Abstract
Purpose
To investigate the outcomes and patient satisfaction at 6-months’ follow-up after switching to faricimab to treat neovascular age-related macular degeneration (nAMD) with a treat-and-extend (TAE) regimen.
Study design
Retrospective observational study.
Methods
Forty-eight consecutive eyes (48 patients) were switched to faricimab to treat nAMD and followed for 6 months on a TAE regimen. The Macular Disease Treatment Satisfaction Questionnaire (MacTSQ) was administered to patients 6 months after the switch.
Results
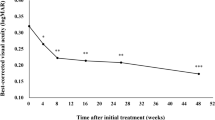
Best-corrected visual acuity (BCVA) was maintained 6 months after the switch, while the mean (± standard error) central foveal thickness 6 months after the switch (272 ± 14 μm) decreased significantly compared to the time of the switch (372 ± 20 μm) (p < 0.001). The interval between injections 6 months after the switch was 10.45 ± 0.44 weeks, a significant extension from 6.72 ± 0.34 weeks at the switch (p = 0.002). The MacTSQ total score (58.8 ± 1.7) in eyes with a BCVA of 20/40 and better 6 months after the switch was significantly higher compared to that in eyes with a BCVA worse than 20/40 (48.2 ± 1.5) (p < 0.001). The MacTSQ total score (56.8 ± 1.8) in eyes in which a 4 weeks extension of the injection interval was achieved was significantly higher than (49.5 ± 1.9) in eyes without (p < 0.001).
Conclusion
Switching to faricimab with a TAE regimen seems to maintain the BCVA and extend the injection interval in patients with nAMD, resulting in enhanced satisfaction.

Similar content being viewed by others
Change history
10 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10384-023-01035-1
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Heier JS, Brown DM, Chong V, Korobelnik J-F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148:43–58.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SVR, Zhang K, Seven-Up Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Wada I, Oshima Y, Shiose S, Kano K, Nakano S, Kaizu Y, et al. Five-year treatment outcomes following intravitreal ranibizumab injections for neovascular age-related macular degeneration in Japanese patients. Graefes Arch Clin Exp Ophthalmol. 2019;257:1411–8.
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-year outcomes of treat-and extend intravitreal aflibercept for exudative age-related macular degeneration. A prospective study. Ophthalmol Retina. 2020;4:767–76.
Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y, et al. Efficacy and safety of intravitreal afliberecept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37:1173–87.
Heier JS, Singh RP, Wykoff CC, Csaky KG, Lai TYY, Loewenstein A, et al. The angiopoietin/Tie pathway in retinal vascular diseases. A review. Retina. 2021;41:1–19.
Heier JS, Khanani AM, Ruiz CQ, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomized, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40.
Mitchell J, Brose LS, Bradley C. Design of a measure of satisfaction with treatment for macular degeneration (MacTSQ). Qual Life Res. 2007;A-120:2.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomized controlled trial. Lancet. 2013;382:1258–67.
Morken TS, Knutsen C, Hanseen MS, Austeng D. Patient satisfaction following a switch from treat-and-extend to observe-and-plan regimen in age-related macular degeneration. BMJ Open. 2022;7: e000930. https://doi.org/10.1136/bmjophth-2021-000930.
Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23:127–40.
Droege KM, Muether PS, Hermann MM, Caramoy A, Viebahn U, Kirchof B, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251:1281–4.
Holz FG, Schmitz-Valckenberg S, Wolf A, Agostini H, Lorenz K, Pielen A, et al. A randomized, open-label, multicenter study of switching to brolucizumab with or without a loading dose for patients with suboptimal anatomically controlled neovascular age-related macular degeneration-the FALCON study. Graefes Arch Clin Exp Ophthalmol. 2022;260:2695–702.
Kitajima Y, Maruyama-Inoue M, Ikeda S, Ito A, Inoue T, Yanagi Y, et al. Short-term outcomes of switching to brolucizumab in Japanese patients with neovascular age-related macular degeneration. Jpn J Ophthalmol. 2022;66:511–7.
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sorbin L, Eliot D, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:29–25.
Gupta OP, Shienbaum G, Patel AH, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–40.
Rush RB, Rush SW. Intravitreal faricimab for aflibercept-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2022;16:4041–6.
Leung EH, Oh DJ, Alderson SE, Bracy J, McLeod M, Prez LI, et al. Initial real-world experience with faricimab in treatment-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2023;17:1287–93.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22.
Acknowledgements
The author thanks Natsuki Kubo, and Haruka Kurabe, CO, for preparation of the data for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
T. Hikichi, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Chugai).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Taiichi Hikichi
About this article
Cite this article
Hikichi, T. Investigation of satisfaction with short-term outcomes after switching to faricimab to treat neovascular age-related macular degeneration. Jpn J Ophthalmol 67, 652–656 (2023). https://doi.org/10.1007/s10384-023-01024-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-023-01024-4