Abstract
Purpose
To evaluate the outer retinal microstructure and visual function after idiopathic macular hole (MH) surgery using internal limiting membrane (ILM) peeling with and without Brilliant Blue G (BBG) staining.
Study design
Retrospective, consecutive case series.
Methods
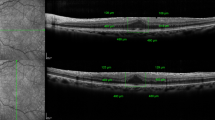
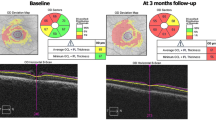
A total of 49 eyes of 47 patients were enrolled: 23 eyes of 23 patients with MH who underwent ILM peeling without dyes (control group) and 26 eyes of 26 patients who underwent BBG staining (BBG group). The lengths of defects of the photoreceptor ellipsoid zone (EZ), external limiting membrane (ELM), and interdigitation zone (IZ) were measured.
Results
The rate of MH closure after initial surgery was 95.6% (22/23 eyes) for the control group versus 100% (26/26 eyes) for the BBG group. In the 48 eyes with MH closure, the recovery rate of ELM deficiency and change in IZ deficiency showed no difference between the groups. The changes in EZ deficiency at 1 and 12 months were greater in the BBG group than in the control group. (P = 0.047 and 0.031). Visual acuity was better in the BBG group than in the control group during 12 months postoperatively (P < 0.001—0.038).
Conclusion
Eyes undergoing BBG-assisted MH surgery achieved faster recovery of the outer retinal structures and greater visual improvement than those of eyes without BBG.




Similar content being viewed by others
Change history
03 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10384-022-00956-7
References
Park DW, Sipperley JO, Sneed SR, Dugel PU, Jacobsen J. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999;106:1392–8.
Mester V, Kuhn F. Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000;129:769–77.
Brooks HL. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000;107:1939–49.
Enaida H, Hisatomi T, Goto Y, Hata Y, Ueno A, Miura M, et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal brilliant blue G. Retina. 2006;26:623–30.
Ueno A, Hisatomi T, Enaida H, Kagimoto T, Mochizuki Y, Goto Y. Biocompatibility of brilliant blue G in a rat model of subretinal injection. Retina. 2007;27:499–504.
Kakurai K, Sugiyama T, Kurimoto T, Oku H, Ikeda T. Involvement of P2X(7) receptors in retinal ganglion cell death after optic nerve crush injury in rats. Neurosci Lett. 2013;534:237–41.
Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, et al. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol. 2011;179:2798–809.
Takeyama A, Imamura Y, Shibata M, Komiya Y, Ishida M. Inner retinal structure and visual function after idiopathic epiretinal membrane surgery with and without brilliant blue G. Jpn J Ophthalmol. 2021;65:689–97.
Scholda C, Wirtitsch M, Hermann B, Unterhuber A, Ergun E, Sattmann H, et al. Ultrahigh resolution optical coherence tomography of macular holes. Retina. 2006;26:1034–41.
Michalewska Z, Michalewski J, Cisiecki S, Adelman R, Nawrocki J. Correlation between foveal structure and visual outcome following macular hole surgery: a spectral optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2008;246:823–30.
Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2009;147:313–18.e1.
Ooka E, Mitamura Y, Baba T, Kitahashi M, Oshitari T, Yamamoto S. Foveal microstructure on spectral-domain optical coherence tomographic images and visual function after macular hole surgery. Am J Ophthalmol. 2011;152:283–90.e1.
Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Significant correlation between visual acuity and recovery of foveal cone microstructures after macular hole surgery. Am J Ophthalmol. 2012;153:111–9.e1.
Ishida M, Ichikawa Y, Higashida R, Tsutsumi Y, Ishikawa A, Imamura Y. Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 2014;157:971–7.
Kumagai K, Ogino N, Furukawa M, Hangai M, Kazama S, Nishigaki S, et al. Retinal thickness after vitrectomy and internal limiting membrane peeling for macular hole and epiretinal membrane. Clin Ophthalmol. 2012;6:679–88.
Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–24.
Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology. 2012;119:1438–46.
Shimozono M, Oishi A, Hata M, Matsuki T, Ito S, Ishida K, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153:698–704. 704.e1.
Wakabayashi T, Oshima Y, Fujimoto H, Murakami Y, Sakaguchi H, Kusaka S, et al. Foveal microstructure and visual acuity after retinal detachment repair: imaging analysis by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116:519–28.
Enaida H, Kumano Y, Ueno A, Yoshida S, Nakao S, Numa S, et al. Quantitative analysis of vitreous and plasma concentrations of brilliant blue g after use as a surgical adjuvant in chromovitrectomy. Retina. 2013;33:2170–4.
Weinberger AW, Kirchhof B, Mazinani BE, Schrage NF. Persistent indocyanine green (ICG) fluorescence 6 weeks after intraocular ICG administration for macular hole surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239:388–90.
Ciardella AP, Schiff W, Barile G, Vidne O, Sparrow J, Langton K, et al. Persistent indocyanine green fluorescence after vitrectomy for macular hole. Am J Ophthalmol. 2003;136:174–7.
Tadayoni R, Paques M, Girmens JF, Massin P, Gaudric A. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology. 2003;110:604–8.
Ashikari M, Ozeki H, Tomida K, Sakurai E, Tamai K, Ogura Y. Retention of dye after indocyanine green-assisted internal limiting membrane peeling. Am J Ophthalmol. 2003;136:172–4.
Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS ONE. 2013;8:e53338. doi:https://doi.org/10.1371/journal.pone.0053338.
Dutot M, Olivier E, Wakx A, Rat P. The role of the P2X7 receptor in ocular stresses: a potential therapeutic target. Vis (Basel). 2017;17:14. doi:https://doi.org/10.3390/vision1020014.
Oh J, Yang SM, Choi YM, Kim SW, Huh K. Glial proliferation after vitrectomy for a macular hole: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013;251:477–84.
Oliveira-Giacomelli Á, Albino CM, de Souza HDN, Corrêa-Velloso J, de Jesus Santos AP, Baranova J, et al. P2Y6 and P2X7 receptor antagonism exerts neuroprotective/neuroregenerative effects in an animal model of Parkinson’s disease. Front Cell Neurosci. 2019;13:476. doi:https://doi.org/10.3389/fncel.2019.00476.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Komiya, None; A. Takeyama, None; M. Shibata, None; Y. Imamura, None; M. Ishida, None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Asuka Takeyama
The original publication has been corrected for revision in Table 3 and Figure 3
About this article
Cite this article
Komiya, Y., Takeyama, A., Shibata, M. et al. Outer retinal microstructure and visual function after macular hole surgery with and without Brilliant Blue G. Jpn J Ophthalmol 66, 534–540 (2022). https://doi.org/10.1007/s10384-022-00942-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-022-00942-z