Abstract
Purpose
To evaluate the serum levels of vascular endothelial growth factor (VEGF) and insulin-like growth factor-1 (IGF-1) after intravitreal injection of aflibercept (IVA) and the transition of aflibercept into systemic circulation in infants with severe retinopathy of prematurity (ROP).
Study design
Prospective study.
Methods
This single-centered prospective cohort study included infants who received IVA for the treatment of type 1 ROP in zone I and posterior zone II. Blood samples were collected before IVA and at 1 day and 1, 2, 4, and 8 weeks after IVA. VEGF, IGF-1 and aflibercept levels were measured.
Results
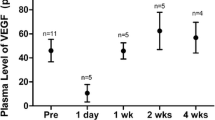
Thirty eyes of 15 infants received IVA of 1 mg/0.025 mL. Serum VEGF levels decreased significantly at 1 day and 1, 2, 4, and 8 weeks after IVA compared with baseline (P < 0.05). Serum aflibercept levels decreased significantly 1, 2, 4, and 8 weeks after IVA compared with the level at 1 day after IVA (P < 0.05) and increased significantly at 1 day, 1, 2, and 4 weeks after IVA compared with the baseline level (P < 0.05). No significant difference was detected between serum IGF-1 levels any time in any infant (P > 0.05).
Conclusion
Serum VEGF levels are suppressed for at least 8 weeks, and aflibercept could be detected in the systemic circulation at 4 weeks after injection. Clinicians should be cautious about changes in systemic VEGF levels and passage of the agent into systemic circulation after IVA in infants.


Similar content being viewed by others
References
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4.
Smith LEH. Pathogenesis of retinopathy of prematurity. Semin Neonatol. 2003;8:469–73.
Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–8.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85.
Fierson WM. Ophthalmology AA of screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142: e20183061.
Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity. Results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–96.
Huang CY, Lien R, Wang NK, Chao AN, Chen KJ, Chen TL, et al. Changes in systemic vascular endothelial growth factor levels after intravitreal injection of aflibercept in infants with retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2018;256:479–87.
Salman AG, Said AM. Structural, visual and refractive outcomes of intravitreal aflibercept injeciton in high-risk prethreshold type 1 retinopathy of prematurity. Ophthal Res. 2015;53:15–20.
Werther K, Christensen IJ, Nielsen HJ. Determination of vascular endothelial growth factor (VEGF) in circulating blood: significance in VEGF in various leucocytes and platelets. Scand J Clin Lab Invest. 2002;62:343–50.
Dittadi R, Meo S, Fabris F, Gasparini G, Contri D, Medici M, et al. Validation of blood collection procedures for the determination of circulating vascular endothelial growth factor (VEGF) in different blood compartments. Int J Biol Markers. 2001;16:87–96.
Sato T, Kusaka S, Shimojo H, Fujikado T. Simultaneous analyses of vitreous levels of 27 cytokines in eyes with retinopathy of prematurity. Ophthalmology. 2009;116:2165–9.
Peirovifar A, Gharehbaghi MM, Gharabaghi PM, Sadeghi K. Vascular endothelial growth factor and insulin-like growth factor-1 in preterm infants with retinopathy of prematurity. Singapore Med J. 2013;54:709–12.
Sato T, Wada K, Arahori H, Kuno N, Imoto K, Shima CI, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33.
Wu WC, Lien R, Liao PJ, Wang NK, Chen YP, Chao AN, et al. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015;133:391–7.
Hoerster R, Muether P, Dahlke C, Mehler K, Oberthür A, Kirchhof B, et al. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013;91:74–5.
Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158:738–44.
Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol. 2015;93:e154–9.
Hirano T, Toriyama Y, Iesato Y, Imai A, Murata T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept or ranibizumab for diabetic macular edema. Retina. 2018;38:1801–8.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–9.
Kennedy KA, Mintz-Hittner HA. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS. 2018;22:61-65.e1.
Lien R, Yu MH, Hsu KH, Liao PJ, Chen YP, Lai CC, et al. Neurodevelopmental outcomes in infants with retinopathy of prematurity and bevacizumab treatment. PloS One. 2016;11: e0148019.
Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137: e20153218.
Zhou Y, Jiang Y, Bai Y, Wen J, Chen L. Vascular endothelial growth factor plasma levels before and after treatment of retinopathy of prematurity with ranibizumab. Graefes Arch Clin Exp Ophthalmol. 2016;254:31–6.
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology. 2007;114:2179–82.
Ternant D, Paintaud G. Pharmacokinetics and concentration-effect relationships of therapeutic monoclonal antibodies and fusion proteins. Expert Opin Biol Ther. 2005;5:S37–47.
Do DV, Rhoades W, Nguyen QD. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina. 2020;40:643–7.
Kaiser PK, Kodjikian L, Korobelnik JF, Winkler J, Torri A, Zeitz O, et al. Systemic pharmacokinetic/pharmacodynamic analysis of intravitreal aflibercept injection in patients with retinal diseases. BMJ Open Ophthalmol. 2019;4: e000185.
Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum vegf and igf-1 in infants with retinopathy of prematurity. Investig Ophthalmol Vis Sci. 2015;56:956–61.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular amd. Br J Ophthalmol. 2014;98:1636–41.
Palmieri TL. Children are not little adults: blood transfusion in children with burn injury. Burns Trauma. 2017;5:24.
Laude A, Tan LE, Wilson CG, Lascaratos G, Elashry M, Aslam T, et al. Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Progr Retinal Eye Res. 2010;29:466–75.
Quintanilla LG, Rodríguez AL, Martínez MG, Garcia CM, Maronas O, Sanjuan VM, et al. Pharmacokinetics of ıntravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics. 2019;11:365.
Kim H, Robinson SB, Csaky KG. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 2009;15:2803–12.
Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, et al. Low IGF-1 suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–8.
Hellström A, Engström E, Hård AL, Wikland KA, Carlsson B, Niklasson A, et al. Postnatal serum insulin-like growth factor i deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–20.
Löfqvist C, Engström E, Sigurdsson J, Hard AL, Niklasson A, Ewald U, et al. Postnatal head growth deficit among premature infants parallels retinopathy of prematurity and insulin-like growth factor-1 deficit. Pediatrics. 2006;117:1930–8.
Acknowledgements
This study was funded in part by University of Health Sciences Bakirkoy Dr. Sadi Konuk Research and Training Hospital, Education Board of Medical Specialties, Istanbul, Turkey. Grant/award numbers: 071218146. The authors would like to thank the hospital staff and parents for their participation in this study. Also, we would like to thank the Scribendi Editing Service for professional medical English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
U. Furuncuoglu, None; A. Vural, None; A. Kural, None; I. U. Onur, None; F. U. Yigit, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Utku Furuncuoglu.
About this article
Cite this article
Furuncuoglu, U., Vural, A., Kural, A. et al. Serum vascular endothelial growth factor, insulin-like growth factor-1 and aflibercept levels in retinopathy of prematurity. Jpn J Ophthalmol 66, 151–158 (2022). https://doi.org/10.1007/s10384-021-00895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00895-9