Abstract
Purpose
To determine whether there are significant correlations between the focal photopic negative response (PhNR), the focal visual sensitivity and the ganglion cell complex (GCC) thickness in glaucomatous eyes.
Study design
Single-center observational study.
Methods
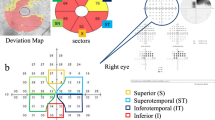
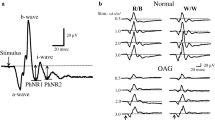
Fifty-two eyes of 52 patients (71.4 ± 9.42 years) with clinically diagnosed open angle glaucoma were studied. Thirty-six age-matched normal subjects served as controls. The focal PhNR of the focal macular electroretinograms (fmERGs) were elicited by a 15° circular, a superior semicircular or an inferior semicircular stimulus centered on the fovea. The thickness of the GCC was measured in the corresponding retinal areas in the spectral-domain optical coherence tomographic images. The visual sensitivities (dB) were measured by microperimetry at the retinal area where the fmERGs were elicited and were converted to liner values (1/Lambert).
Results
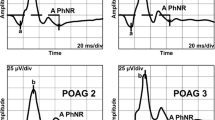
The focal PhNR amplitudes were significantly correlated with the visual sensitivities of the full-circle (R = 0.532), the superior (R = 0.530) and inferior (R = 0.526) semicircular responses (P < 0.0001). The GCC thickness was correlated with the visual sensitivities in the same areas with stronger correlations (R = 0.700, 0.759 and 0.650, respectively; P < 0.0001). The focal PhNR amplitudes were proportionally reduced with the thinning of the GCC thickness (R = 0.494, 0.518 and 0.511, respectively; P < 0.0001).
Conclusions
The significant correlations between the focal PhNR amplitudes, the focal visual sensitivities and the GCC thickness indicate that these may be good biomarkers to track the changes in the physiology and anatomy of the macular area in glaucomatous eyes.





Similar content being viewed by others
References
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–36.
Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42:514–22.
Gotoh Y, Machida S, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004;122:341–6.
Machida S, Gotoh Y, Tanaka M, Tazawa Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol. 2004;137:938–40.
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA. Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci. 2004;45:3827–37.
Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, et al. Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci. 2007;48:820–4.
Ueno S, Kondo M, Piao CH, Ikenoya K, Miyake Y, Terasaki H. Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG-assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci. 2006;47:3545–9.
Chen H, Wu D, Huang S, Yan H. The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol. 2006;113:53–9.
Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006;50:367–73.
Moon CH, Hwang SC, Ohn YH, Park TK. The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci. 2011;52:7966–73.
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008;49:2201–7.
Tamada K, Machida S, Yokoyama D, Kurosaka D. Photopic negative response of full-field and focal macular electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol. 2009;53:608–14.
Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK. Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci. 2011;52:8527–33.
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ. The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci. 2012;53:1315–23.
Machida S. Clinical applications of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. 2012;2012: 397178. https://doi.org/10.1155/2012/397178.
Miyake Y, Yanagida K, Kondo K, Ota I. Subjective scotometry and recording of local electroretinogram and visual evoked response. System with television monitor of the fundus. Jpn J Ophthalmol. 1981;25:439–48.
Miyake Y. Studies of local macular ERG. Nippon Ganka Gakkai Zassh. 1988;92:1419–49 (in Japanese).
Machida S, Toba Y, Ohtaki A, Gotoh Y, Kaneko M, Kurosaka D. Photopic negative response of focal electroretinograms in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008;49:5636–44.
Kurimoto Y, Kondo M, Ueno S, Sakai T, Machida S, Terasaki H. Asymmetry of focal macular photopic negative responses (PhNRs) in monkeys. Exp Eye Res. 2009;88:92–8.
Nakamura H, Hangai M, Mori S, Hirose F, Yoshimura N. Hemispherical focal macular photopic negative response and macular inner retinal thickness in open-angle glaucoma. Am J Ophthalmol. 2011;151:494–506.
Tamada K, Machida S, Oikawa T, Miyamoto H, Nishimura T, Kurosaka D. Correlation between photopic negative response of focal electroretinograms and local loss of retinal neurons in glaucoma. Curr Eye Res. 2010;35:155–64.
Machida S, Kaneko M, Kurosaka D. Regional variations in correlation between photopic negative response of focal electroretinograms and ganglion cell complex in glaucoma. Curr Eye Res. 2015;40:439–49.
Kaneko M, Machida S, Hoshi Y, Kurosaka D. Alterations of photopic negative response of multifocal electroretinogram in patients with glaucoma. Curr Eye Res. 2015;40:77–86.
Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–30.
Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107:453–64.
Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000;41:2205–11.
Machida S, Tamada K, Oikawa T, Yokoyama D, Kaneko M, Kurosaka D. Sensitivity and specificity of photopic negative response of focal electoretinogram to detect glaucomatous eyes. Br J Ophthalmol. 2010;94:202–8.
Machida S, Tamada K, Oikawa T, Gotoh Y, Nishimura T, Kaneko M, et al. Comparison of photopic negative response of full-field and focal electroretinograms in detecting glaucomatous eyes. J Ophthalmol. 2011;2011:564131.
Rao HL, Januwada M, Hussain RS, Pillutla LN, Begum VU, Chaitanya A, et al. Comparing the structure-function relationship at the macula with standard automated perimetry and microperimetry. Invest Ophthalmol Vis Sci. 2015;56:8063–8.
Harwerth RS, Carter-Dawson L, Shen F, Smith EL 3rd, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–50.
Harwerth RS, Carter-Dawson L, Smith EL 3rd, Barnes G, Holt WF, Crawford ML. Neural losses correlated with visual losses in clinical perimetry. Invest Ophthalmol Vis Sci. 2004;45:3152–60.
Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006;124:853–9.
Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL 3rd. The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48:763–73.
Hood DC, Greenstein VC, Odel JG, Zhang X, Ritch R, Liebmann JM, et al. Visual field defects and multifocal visual evoked potentials: evidence of a linear relationship. Arch Ophthalmol. 2002;120:1672–81.
Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci. 2007;48:3662–8.
Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710.
Scuderi G, Fragiotta S, Scuderi L, Iodice CM, Perdicchi A. Ganglion cell complex analysis in glaucoma patients: what can it tell us? Eye Brain. 2020;12:33–44.
Frishman LJ. Origin of the electroretinogram. In: Heckenlively JR, Arden GB, editors. Principles and practice of clinical electrophysiology of vision. 2nd ed. Cambridge: Massachusetts Institute of Technology; 2006. p. 139–83.
Viswanathan S, Frishman LJ. Evidence that negative potentials in the photopic electroretinograms of cats and primates depend upon spiking activity of retinal ganglion cell axons. Soc Neurosci Abstr. 1997;23:1024.
Tanihara H, Hangai M, Sawaguchi S, Abe H, Kageyama M, Nakazawa F, et al. Up-regulation of glial fibrillary acidic protein in the retina of primate eyes with experimental glaucoma. Arch Ophthalmol. 1997;115:752–6.
Machida S, Kondo M, Jamison JA, Khan NW, Kononen LT, Sugawara T, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci. 2000;41:3200–9.
Hood DC, Benimoff NI, Greenstein VC. The response range of the blue-cone pathways: a source of vulnerability to disease. Invest Ophthalmol Vis Sci. 1984;25:864–7.
Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, et al. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011;129:1529–36.
Hood DC, Raza AS, de Moraes CG, Odel JG, Greenstein VC, Liebmann JM, et al. Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:940–6.
Turpin A, Chen S, Sepulveda JA, McKendrick AM. Customizing structure-function displacements in the macula for individual differences. Invest Ophthalmol Vis Sci. 2015;56:5984–9.
Frishman L, Sustar M, Kremers J, McAnany JJ, Sarossy M, Tzekov R, et al. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol. 2018;136:207–11.
Acknowledgements
This study was supported by JSPS KAKENHI Grant Number 18K09420 (SM). We thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute for discussions and editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Ishizuka, None; S. Machida, None; Y. Hara, None; A. Tada, None; S. Ebihara, None; M. Gonmori, None; T. Nishimura, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Shigeki Machida
About this article
Cite this article
Ishizuka, M., Machida, S., Hara, Y. et al. Significant correlations between focal photopic negative response and focal visual sensitivity and ganglion cell complex thickness in glaucomatous eyes. Jpn J Ophthalmol 66, 41–51 (2022). https://doi.org/10.1007/s10384-021-00886-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00886-w