Abstract
Purpose
To evaluate the changes in the posterior ocular structures and glaucoma susceptibility in patients with hemifacial spasm (HFS).
Study design
Prospective observational clinical study.
Methods
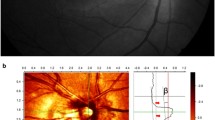
This study included 46 long-standing HFS patients with a minimum follow-up of 12 months. The participants' eyes were divided into three groups: (1) 46 affected eyes of patients with clinical HFS; (2) 46 unaffected fellow eyes and, (3) 46 eyes of healthy sex and age-matched controls. All participants were assessed by a detailed clinical examination and optical coherence tomography (OCT) with enhanced depth imaging (EDI). EDI–OCT images were binarized using ImageJ software. Peripapillary retinal nerve fiber layer (RNFL), ganglion cell complex (GCC), choroidal thickness (CT), and choroidal vascularity index (CVI) were used to compare the structural characteristics of the affected eyes with fellow and control eyes. The CT measurements were performed from the subfoveal and locations at 750 μm and 3000 μm intervals from the foveal center, and the average of CT measurements at 5 locations was accepted as mean CT.
Results
The demographic and clinical characteristics among the groups were similar (for all, P > 0.05). Mean peripapillary RNFL thickness of the inferior quarter was significantly lower in affected eyes, compared to fellow eyes (P = 0.023) and control eyes (P = 0.040). Mean GCC thickness significantly decreased in affected eyes, compared to fellow eyes (P = 0.019) and healthy controls (P = 0.008). Mean CT thickness significantly decreased in affected eyes, compared to fellow eyes (P = 0.002) and healthy controls (P < 0.001). Mean subfoveal CVI (65.94% ± 1.46) was found to be significantly thinner than the unaffected fellow (68.19% ± 1.84, P = 0.011) eyes and control eyes (67.23% ± 0.84, P = 0.044).
Conclusions
This study's outcomes show that long-standing HFS is associated with glaucoma-associated morphological OCT findings and decreased both CT and subfoveal choroidal vascularity. These findings may be related to the fact that the posterior ocular structures are affected by long-lasting paroxysmal orbicularis contractions.

Similar content being viewed by others
References
Ross AH, Elston JS, Marion MH, Malhotra R. Review and update of involuntary facial movement disorders presenting in the ophthalmological setting. Surv Ophthalmol. 2011;56:54–67.
Girard N, Poncet M, Caces F, Tallon Y, Chays A, Martin-Bouyar P, et al. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997;39:46–51.
Yaltho TC, Jankovic J. The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26:1582–92.
Ababneh OH, Cetinkaya A, Kulwin DR. Long-term efficacy and safety of botulinum toxin A injections to treat blepharospasm and hemifacial spasm. Clin Exp Ophthalmol. 2014;42:254–61.
Osaki T, Osaki MH, Osaki TH, Hirai FE, Campos M. Differences in corneal parameters between affected and normal contralateral eyes in patients with hemifacial spasm treated with botulinum toxin-a: outcomes during one complete treatment cycle. Cornea. 2016;35:220–5.
Osaki T, Osaki MH, Osaki TH, Hirai FE, Nallasamy N, Campos M. Influence of involuntary eyelid spasms on corneal topographic and eyelid morphometric changes in patients with hemifacial spasm. Br J Ophthalmol. 2016;100:963–70.
Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82:637–40.
Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, Li G, Gaasterland D, et al. Predictive factors for glaucomatous visual field progression in the advanced glaucoma intervention study. Ophthalmology. 2004;111:1627–35.
Killer HE, Rüst O, Müller O, Flammer J. Unilateral glaucomatous damage in a patient with hemifacial spasm. Ophthalmologica. 1999;213:273–5.
Cicik E, Yildirim R, Arici C, Dikkaya F, Arslan OS. Effect of hemifacial spasm on intraocular pressure measurement. J Ophthalmol. 2018;2018:3621215.
Low JR, Wong CW, Loo JL, Milea D, Perera SA, Lee YF, et al. Evaluation of intraocular pressure after water drinking test in patients with unilateral hemifacial spasm. Clin Ophthalmol. 2020;14:1675–80.
Jankovic J, Kenney C, Grafe S, Goertelmeyer R, Comes G. Relationship between various clinical outcome assessments in patients with blepharospasm. Mov Disord. 2009;24:407–13.
Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, et al. Luminal and stroma areas of choroid determined by binarization method of optical coherence tomographic images. Am Ophthalmol. 2015;159:1123-31.e1.
Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090.
Kenney C, Jankovic J. Botulinum toxin in the treatment of blepharospasm and hemifacial spasm. J Neural Transm (Vienna). 2008;115:585–91.
Saunders RA, Helveston EM, Ellis FD. Differential intraocular pressure in strabismus diagnosis. Ophthalmology. 1981;88:59–70.
Gandhi PD, Gürses-Ozden R, Liebmann JM, Ritch R. Attempted eyelid closure affects intraocular pressure measurement. Am J Ophthalmol. 2001;131:417–20.
Jamal KN, Gürses-Ozden R, Liebmann JM, Ritch R. Attempted eyelid closure affects intraocular pressure measurement in open-angle glaucoma patients. Am J Ophthalmol. 2002;134:186–9.
Klamann MK, Grünert A, Maier AK, Gonnermann J, Joussen AM, Huber KK. Comparison of functional and morphological diagnostics in glaucoma patients and healthy subjects. Ophthalmic Res. 2013;49:192–8.
Agrawal R, Wei X, Goud A, Vupparaboina KK, Jana S, Chhablani J. Influence of scanning area on choroidal vascularity index measurement using optical coherence tomography. Acta Ophthalmol. 2017;95:e770–5.
Park Y, Cho KJ. Choroidal vascular index in patients with open angle glaucoma and preperimetric glaucoma. PLoS ONE. 2019;14(3):e0213336.
Jo YH, Sung KR, Shin JW. Comparison of peripapillary choroidal microvasculature dropout in primary open-angle, primary angle-closure, and pseudoexfoliation glaucoma. J Glaucoma. 2020;29:1152–7.
Waliszek-Iwanicka A, Waliszek M, Banach M, Rysz J, Gos R. Assessment of blood flow in posterior ciliary arteries and its correlation with intraocular and arterial blood pressures in patients with open angle glaucoma. Med Sci Monit. 2010;16(10):CR5501–9.
Wong VHY, Zhao D, Bui BV, Millar CJ, Nguyen CTO. Increased episcleral venous pressure in a mouse model of circumlimbal suture induced ocular hypertension. Exp Eye Res. 2021;202:8348.
Ethier CR, Yoo P, Berdahl JP. The effects of negative periocular pressure on intraocular pressure. Exp Eye Res. 2020;191:107928.
Reiner A, Fitzgerald MEC, Del Mar N, Li C. Neural control of choroidal blood flow. Prog Retin Eye Res. 2018;64:96–130.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
C. Ozsaygili, None; N. Bayram, None; S. Kılıc, None; İ. Perente, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Cemal Özsaygili
About this article
Cite this article
Ozsaygili, C., Bayram, N., Kılıc, S. et al. Posterior ocular structural changes and glaucoma susceptibility in patients with hemifacial spasm. Jpn J Ophthalmol 65, 827–835 (2021). https://doi.org/10.1007/s10384-021-00876-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00876-y