Abstract
Purpose
To investigate the 4-year outcome of aflibercept treatment using a treat-and-extend (TAE) regimen for recurrent neovascular age-related macular degeneration (AMD).
Study design
Retrospective observational study.
Methods
Data of eyes with recurrent AMD previously treated with anti-vascular endothelial growth factor agents or photodynamic therapy and had started aflibercept treatment using a TAE regimen for the first time were collected. Best-corrected visual acuity (BCVA), intervals of treatments, the presence of exudation, central foveal thickness (CFT), and central choroidal thickness (CCT) were analyzed.
Results
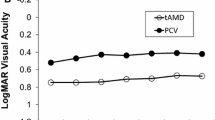
Of 47 consecutive eyes, 30 of the 47 eyes completed a 4-year follow-up. The mean BCVA (logMAR) was sustained over the 4 years (0.37 at baseline, 0.36 at 1 year, 0.36 at 2 years, 0.41 at 3 years, and 0.43 at 4 years, P = 0.21). Of the 30 eyes that completed the follow-up, BCVA of two eyes deteriorated by 0.3 logMAR or more at 4 years. At 4 years, 67% of eyes had extended treatment intervals to > 8 weeks, and 47% of eyes had extended intervals to > 12 weeks. Exudative changes in the macula, seen in all eyes at baseline, were only seen in 50% of the eyes at 4 years. The mean CFT and CCT decreased significantly at 4 years from 332 μm to 248 μm and from 218 μm to 183 μm, respectively.
Conclusion
In clinical settings, aflibercept treatment using a TAE regimen may successfully maintain visual acuity for up to 4 years even in recurrent cases of AMD.




Similar content being viewed by others
References
Morizane Y, Morimoto N, Fujiwara A, Kawasaki R, Yamashita H, Ogura Y, et al. Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol. 2019;63:26–33.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65.
Group C, Martin DF, Maguire MG, Ying G-S, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Singer MA, Awh CC, Sadda SV, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Group CoA-rMDTTCR, Maguire MG, Martin DF, Ying G-SS, Jaffe GJ, Daniel E, et al. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123:1751–61.
Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration trex-amd 1-year results. Ophthalmology. 2015;122:2514–22.
Engelbert M, Zweifel SA, Freund KB. Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina. 2010;30:1368–75.
Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143:679–80.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology. 2010;117:2134–40.
Wykoff CC, Ou WC, Brown DM, Croft DE, Wang R, Payne JF, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration 2-year results of the trex-amd study. Ophthalmol Retina. 2017;1:314–21.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser P, Nguyen Q, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Eleftheriadou M, Gemenetzi M, Lukic M, Sivaprasad S, Hykin PG, Hamilton RD, et al. Three-year outcomes of aflibercept treatment for neovascular age-related macular degeneration: evidence from a clinical setting. Ophthalmol Ther. 2018;7:361–8.
Morimoto M, Matsumoto H, Mimura K, Akiyama H. Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1891–7.
Matsumoto H, Hiroe T, Morimoto M, Mimura K, Ito A, Akiyama H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn J Ophthalmol. 2018;62:144–50.
Barthelmes D, Nguyen V, Daien V, Campain A, Walton R, Guymer R, et al. Two year outcomes of “treat and extend” intravitreal therapy using aflibercept preferentially for neovascular age-related macular degeneration. Retina. 2018;38:20–8.
Traine PG, Pfister IB, Zandi S, Spindler J, Garweg JG. Long-term outcome of intravitreal aflibercept treatment for neovascular age-related macular degeneration using a “treat-and-extend” regimen. Ophthalmol Retina. 2019;3:393–9.
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156:29–35.
Singh RP, Srivastava S, Ehlers JP, Bedi R, Schachat AP, Kaiser PK. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014;98:i22–7.
Barthelmes D, Campain A, Nguyen P, Arnold JJ, McAllister IL, Simpson JM, et al. Effects of switching from ranibizumab to aflibercept in eyes with exudative age-related macular degeneration. Br J Ophthalmol. 2016;100:1640–5.
Kvannli L, Krohn J. Switching from pro re nata to treat-and-extend regimen improves visual acuity in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95:678–82.
Giannakaki-Zimmermann H, Ebneter A, Munk MR, Wolf S, Zinkernagel MS. Outcomes when switching from a pro re nata regimen to a treat and extend regimen using aflibercept in neovascular age-related macular degeneration. Ophthalmologica. 2017;236:201–6.
Spooner K, Hong T, Wijeyakumar W, Chang AA. Switching to aflibercept among patients with treatment-resistant neovascular age-related macular degeneration: a systematic review with meta-analysis. Clin Ophthalmol. 2017;11:161–77.
Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol. 2016;100:914–7.
Augsburger M, Sarra G-MM, Imesch P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 2019;257:1889–95.
Adrean SD, Chaili S, Grant S, Pirouz A. Recurrence rate of choroidal neovascularization in neovascular age-related macular degeneration managed with a treat–extend–stop protocol. Ophthalmol Retina. 2018;2:225–30.
Nguyen V, Vaze A, Fraser-Bell S, Arnold J, Essex RW, Barthelmes D, et al. Outcomes of suspending vegf inhibitors for neovascular age-related macular degeneration when lesions have been inactive for 3 months. Ophthalmol Retina. 2019;3:623–8.
Hosokawa M, Morizane Y, Hirano M, Kimura S, Kumase F, Shiode Y, et al. One-year outcomes of a treat-and-extend regimen of intravitreal aflibercept for polypoidal choroidal vasculopathy. Jpn J Ophthalmol. 2017;61:150–8.
Yamamoto A, Okada AA, Nakayama M, Yoshida Y, Kobayashi H. One-year outcomes of a treat-and-extend regimen of aflibercept for exudative age-related macular degeneration. Ophthalmologica. 2017;237:139–44.
Spaide RF, Jaffe GJ, Sarraf D, Freund BK, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data. Ophthalmology. 2019;127:616–36.
Suzuki M, Nagai N, Izumi-Nagai K, Shinoda H, Koto T, Uchida A, et al. Predictive factors for non-response to intravitreal ranibizumab treatment in age-related macular degeneration. Br J Ophthalmol. 2014;98:1186–91.
Nakano Y, Kataoka K, Takeuchi J, Fujita A, Kaneko H, Shimizu H, et al. Vascular maturity of type 1 and type 2 choroidal neovascularization evaluated by optical coherence tomography angiography. PLoS ONE. 2019;14:e0216304.
Okada M, Kandasamy R, Chong EW, McGuiness M, Guymer RH. The treat-and-extend injection regimen versus alternate dosing strategies in age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2018;192:184–97.
Ito A, Matsumoto H, Morimoto M, Mimura K, Akiyama H. Two-year outcomes of a treat-and-extend regimen using intravitreal aflibercept injections for typical age-related macular degeneration. Ophthalmologica. 2017;238:236–42.
Maruko I, Ogasawara M, Yamamoto A, Itagaki K, Hasegawa T, Arakawa H, et al. Two-year outcomes of treat-and-extend intravitreal aflibercept for exudative age-related macular degeneration: a prospective study. Ophthalmol Retina. 2020;4:767–76.
Acknowledgements
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Y. Tsunekawa, None; K. Kataoka, Honorarium for Lecturing (Novartis, Santen, Bayer, Senju); K. Asai, None; Y. Ito, Honorarium for Lecturing(Aichi Ophthalmologists Association, Bayer, Canon, ZEISS, Kowa, Novartis, Okazaki City Medical Association, Pfizer, Santen); H. Terasaki, Grant, Honorarium for Lecturing(Otsuka, Kowa, Santen, Senju, Alcon, Novartis, Wakamoto), Honorarium for Lecturing, Consultant fee, Travel fee(Bayer), Rohto Award Selection Committee(ROHTO), Honorarium for Lecturing (ZEISS), Grant (HOYA).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Keiko Kataoka
About this article
Cite this article
Tsunekawa, Y., Kataoka, K., Asai, K. et al. Four-year outcome of aflibercept administration using a treat-and-extend regimen in eyes with recurrent neovascular age-related macular degeneration. Jpn J Ophthalmol 65, 69–76 (2021). https://doi.org/10.1007/s10384-020-00783-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00783-8