Abstract
Purpose
Omidenepag isopropyl (OMDI) is the prodrug of omidenepag, a selective, non-prostaglandin, prostanoid EP2 receptor agonist, which has been shown to lower intraocular pressure (IOP) in patients with glaucoma and ocular hypertension (OHT). This study evaluated the efficacy and safety of OMDI ophthalmic solution 0.002% in patients with primary open-angle glaucoma or OHT who were non-/low responders to latanoprost.
Study design
Open-label, multicenter, Phase 3 study (NCT02822742).
Methods
Following 1–4-week washout, patients were treated with latanoprost ophthalmic solution 0.005% during an 8-week run-in period. Patients with ≤15% IOP reduction at the end of the run-in (indicating non-/low response) received OMDI 0.002% (one drop once daily for 4 weeks). The primary endpoint was the change from baseline in mean diurnal IOP at Week 4.
Results
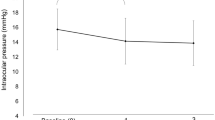
In total, 26 patients were treated with OMDI; two withdrew owing to lack of efficacy. The mean diurnal IOP at baseline (end of latanoprost run-in) was 23.1 mmHg (7.6% IOP reduction from end of washout) indicating non-/low response to latanoprost. After 4 weeks of OMDI treatment, mean diurnal IOP was significantly reduced from baseline (−2.99 mmHg; P < 0.0001). No serious adverse events were reported. Adverse events occurred in five patients (19.2%); adverse drug reactions (anterior chamber cell, conjunctival hyperemia, and erythema of eyelid) occurred in two patients (7.7%) and were mild in severity.
Conclusions
In this study, OMDI 0.002% demonstrated a clinically significant reduction in IOP and was well tolerated in patients with primary open-angle glaucoma and OHT who were non-/low responders to latanoprost.




Similar content being viewed by others
References
Quigley HA, Broman A. Glaucoma. Lancet. 2011;377:1367–77.
Chader GJ. Key needs and opportunities for treating glaucoma. Invest Ophthalmol Vis Sci. 2012;53:2456–60.
Mantravadi AV, Vadhar N. Glaucoma. Prim Care. 2015;42:437–49.
Cheema A, Chang RT, Shrivastava A, Singh K. Update on the medical treatment of primary open-angle glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5:51–8.
European Glaucoma Society. Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 3: treatment principles and options. Br J Ophthalmol. 2017;101:130–95.
van der Valk R, Webers CA, Schouten JS, Zeegers MP, Hendrikse F, Prins MH. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: a meta-analysis of randomized clinical trials. Ophthalmology. 2005;112:1177–85.
Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6:45–52.
Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606.
electronic Medicines Compendium (eMC). SAFLUTAN 15 micrograms/mL eye drops, solution: summary of product characteristics (SmPC) [Internet]. 2017 [cited 2019 May 2]. https://www.medicines.org.uk/emc/product/5115/pil
Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22:626–31.
Inoue K. Managing adverse effects of glaucoma medications. Clin Ophthalmol. 2014;8:903–13.
electronic Medicines Compendium (eMC). Travatan: summary of product characteristics (SmPC) [Internet]. 2017 [cited 2019 May 2]. https://www.medicines.org.uk/emc/product/1556/smpc
electronic Medicines Compendium (eMC). Latanoprost 0.005% w/v eye drops solution: summary of product characteristics (SmPC) [Internet]. 2018 [cited 2019 May 2]. https://www.medicines.org.uk/emc/product/5974/smpc
Maruyama K, Shirato S, Tsuchisaka A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J Glaucoma. 2014;23:160–3.
Yanagi M, Kiuchi Y, Yuasa Y, Yoneda T, Sumi T, Hoshikawa Y, et al. Association between glaucoma eye drops and hyperemia. Jpn J Ophthalmol. 2016;60:72–7.
Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy with a latanoprost ophthalmic solution. Eye (Lond). 2014;28:1446–51.
Miki T, Naito T, Fujiwara M, Araki R, Kiyoi R, Shiode Y, et al. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. Bhattacharya S, editor. PLoS ONE. 2017;12:e0181550.
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13.
Schmier JK, Covert DW, Hulme-Lowe CK. Adjunctive therapy patterns in glaucoma patients using prostaglandin analogs. Clin Ophthalmol. 2014;8:1097–104.
Schmier JK, Lau EC, Covert DW. Two-year treatment patterns and costs in glaucoma patients initiating treatment with prostaglandin analogs. Clin Ophthalmol. 2010;4:1137–43.
electronic Medicines Compendium (eMC). Timolol eye drops 0.5%: summary of product characteristics (SmPC) [Internet]. 2016 [cited 2018 Oct 3]. https://www.medicines.org.uk/emc/product/4053/smpc
electronic Medicines Compendium (eMC). Brimonidine tartrate 0.2% w/v eye drops: summary of product characteristics (SmPC) [Internet]. 2014 [cited 2018 Oct 3]. https://www.medicines.org.uk/emc/product/3426/smpc
electronic Medicines Compendium (eMC). COSOPT 20 mg/ml + 5 mg/ml, eye drops, solution: summary of product characteristics (SmPC) [Internet]. 2018 [cited 2019 Apr 8]. https://www.medicines.org.uk/emc/product/5113/smpc
U.S. Food & Drug Administration (FDA). AZOPT® (brinzolamide opthalmic suspension) 1% sterile topical opthalmic drops package insert [Internet]. 2015 [cited 2019 Nov 7]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020816s019lbl.pdf
electronic Medicines Compendium (eMC). AZOPT® eye drops, suspension: summary of product characteristics [Internet]. 2018 [cited 2019 Nov 7]. https://www.medicines.org.uk/emc/product/3819
Merck. TRUSOPT® (dorzolamide hydrochloride ophthalmic solution) 2%: highlights of prescribing information. [Internet]. 2014 [cited 2018 Aug 30]. https://www.merck.com/product/usa/pi_circulars/t/trusopt/trusopt_pi.pdf
Allergan. ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%. Prescribing information. [Internet]. 2016 [cited 2018 Aug 30]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020613s031lbl.pdf
Ikeda Y, Mori K, Ishibashi T, Naruse S, Nakajima N, Kinoshita S. Latanoprost nonresponders with open-angle glaucoma in the Japanese population. Jpn J Ophthalmol. 2006;50:153–7.
Kirihara T, Taniguchi T, Yamamura K, Iwamura R, Yoneda K, Odani-Kawabata N, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59:145–53.
Santen Pharmaceutical Co., Ltd, Ube Industries. Press release. Santen and Ube industries announce receipt of manufacturing and marketing approval for glaucoma and ocular hypertension treatment EYBELIS ophthalmic solution 0.002% in Japan [Internet]. 2018 [cited 2019 May 20]. https://www.ube-ind.co.jp/ube/en/news/2018/20180921_01.html
Santen Pharmaceutical Co., Ltd. Pipeline development status (clinical stage) [Internet]. 2020 [cited 2020 Mar 10]. https://www.santen.com/en/rd/#Pipeline
Aihara M, Lu F, Kawata H, Tanaka Y, Yamamura K, Iwamura R, et al. A selective agonist for EP2 receptor in healthy volunteers: safety, pharmacokinetics and pharmacodynamics of omidenepag isopropyl. In: Poster presented at the 6th World Glaucoma Congress (WGC), Helsinki, Finland, 28 June–1 July 2017. 2017 p. (Abstract P-WT-119).
Fuwa M, Toris CB, Fan S, Taniguchi T, Ichikawa M, Odani-Kawabata N, et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocul Pharmacol Ther. 2018;34:531–7.
Toris CB, Camras CB, Yablonski ME, Brubaker RF. Effects of exogenous prostaglandins on aqueous humor dynamics and blood-aqueous barrier function. Surv Ophthalmol. 1997;41(Suppl. 2):S69–75.
Weinreb RN, Toris CB, Gabelt BT, Lindsey JD, Kaufman PL. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol. 2002;47(Suppl. 1):S53–64.
Aihara M, Lu F, Kawata H, Iwata A, Liu K, Odani-Kawabata N, et al. Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28:375–85.
Yamagishi-Kimura R, Honjo M, Aihara M. Contribution of prostanoid FP receptor and prostaglandins in transient inflammatory ocular hypertension. Sci Rep. 2018;8:11098.
Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34:413–23.
Santen Pharmaceutical Co., Ltd. EYBELIS® ophthalmic solution 0.002% package insert [Japanese] [Internet]. 2018 [cited 2019 Mar 1]. https://www.santen.co.jp/medical-channel/di/tenpu/DD067_eybelis.pdf#
Rossetti L, Gandolfi S, Traverso C, Montanari P, Uva M, Manni G, et al. An evaluation of the rate of nonresponders to latanoprost therapy. J Glaucoma. 2006;15:238–43.
Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22:1–21.
Toris CB, Gleason ML, Camras CB, Yablonski ME. Effects of brimonidine on aqueous humor dynamics in human eyes. Arch Ophthalmol. 1995;113:1514–7.
Asia-Pacific Glaucoma Society. Asia-Pacific glaucoma guidelines [Internet]. Third Edn. Amsterdam, The Netherlands: Kugler Publications; 2016 [cited 2020 Jan 7]. https://apglaucomasociety.org/Public/Resources/APGG/Public/Resources/APGG.aspx?hkey=8b82cd44-a24c-4c56-8694-19aadef206b0
Acknowledgements
Medical writing was provided by Sandra Callagy, PhD, and Jennifer Mitchell, PhD, both of Helios Medical Communications, Cheshire, UK, which was funded by Santen. The study was sponsored by Santen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Aihara, Grant, Research support, Financial support, Consultant fee, Lecture fee (Alcon), Financial support, Lecture fee (Johnson & Johnson), Financial support, Consultant fee, Lecture fee (Crewt Medical Systems, Glaukos, Kowa, Otsuka), Consultant fee, Lecture fee (HOYA, IRIDEX), Financial support, Lecture fee (Nitten, Novartis, Ono), Grant, Research support, Financial support, Consultant fee, Lecture fee, Travel fee (Pfizer), Financial support, Consultant fee, Lecture fee, Travel fee (Santen, Senju), Grant, Research support, Financial support, Lecture fee (Sato), Financial support, Lecture fee (TOMEY), Financial support, Consultant fee (Wakamoto), Consultant fee, Lecture fee (InnFocus), Lecture fee (NIDEK, Ivantis, Canon, ZEISS), Consultant fee (Astellas); A. Ropo, Employee (Santen); F. Lu, Employee (Santen); H. Kawata, Employee (Santen); A. Iwata, Employee (Santen); N. O. Kawabata, Employee (Santen); N. Shams, Employee (Santen).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Auli Ropo
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Aihara, M., Ropo, A., Lu, F. et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol 64, 398–406 (2020). https://doi.org/10.1007/s10384-020-00748-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00748-x