Abstract
Purpose
To determine whether photoreceptor degeneration in transgenic (Tg) rabbits carrying the Pro347Leu rhodopsin mutation alters the neural activity of the middle and inner retinal neurons.
Methods
Multifocal electroretinograms (mfERGs) were recorded from eight 12-week-old Tg rabbits both before and after intravitreal injection of the following: tetrodotoxin citrate (TTX), N-methyl-dl-aspartic acid (NMDA), 2-amino-4-phosphonobutyric acid (APB), and cis-2,3-piperidine-dicarboxylic acid (PDA). Digital subtraction of the mfERGs recorded after the drugs were administered from those recorded before was used to extract the components that were eliminated by these drugs. Eight agematched, wild-type (WT) rabbits were studied with the same protocol.
Results
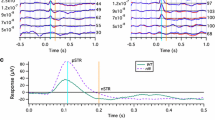
There was no reduction in the amplitude of the cone photoreceptor response of the mfERGs in Tg rabbits. Both the first positive and the first negative waves of the ON-bipolar cell responses were significantly larger in the Tg than in the WT rabbits. Late negative waves of the ON-bipolar cell response were recorded only in the WT rabbits. The first negative wave of the inner retinal responses was larger in the Tg than in the Wt rabbits. The late positive waves were seen mainly in the WT rabbits.
Conclusions
The ON-bipolar cell and inner retinal responses were altered at the early stage of photoreceptor degeneration in Tg rabbits despite the preservation of the cone photoreceptor responses.
Similar content being viewed by others
References
Berson EL, Rosner B, Sandberg MA, et al. Ocular findings in patients with autosomal dominant retinitis pigmentosa and rhodopsin, proline-347-leucine. Am J Ophthalmol 1991;111:614–623.
Oh KT, Longmuir R, Oh DM, et al. Comparison of the clinical expression of retinitis pigmentosa associated with rhodopsin mutations at codon 347 and codon 23. Am J Ophthalmol 2003;136:306–313.
Sung CH, Makino C, Baylor D, Nathans J. A rhodopsin gene mutation responsible for autosomal dominant retinitis pigmentosa results in a protein that is defective in localization to the photoreceptor outer segment. J Neurosci 1994;14:5818–5833.
Green ES, Menz MD, LaVail MM, Flannery JG. Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 2000;41:1546–1553.
Peng YW, Hao Y, Petters RM, Wong F. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci 2000;3:1121–1127.
Kondo M, Sakai T, Komeima K, et al. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci 2009;50:1371–1377.
Sakai T, Kondo M, Ueno S, et al. Supernormal ERG oscillatory in transgenic rabbit with rhodopsin P347L mutation and retinal degeneration. Invest Ophthalmol Vis Sci 2009;50:4402–4409.
Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol 1964;47:965–974.
Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev 1974;54:813–889.
Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol 1996;75:1878–1893.
Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 2002;43:1673–1685.
Ng YF, Chan HH, Chu PH, et al. Multifocal electroretinogram in rhodopsin P347L transgenic pigs. Invest Ophthalmol Vis Sci 2008;49:2208–2215.
Slaughter MM, Miller RF. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci 1983;3:1701–1711.
Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retinal research. Science 1981;211:182–185.
Slaughter MM, Miller RF. An excitatory amino acid antagonist blocks cone input to sign-conserving second-order retinal neuron. Science 1983;219:1230–1232.
Gao H, Pennesi ME, Qiao X, et al. Intravitreal moxifloxacin: retinal safety study with electroretinography and histopathology in animal models. Invest Ophthalmol Vis Sci 47;2006:1606–1611.
Sieving PA, Murayama K, Narrendorp F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons shaping the b-wave. Vis Neurosci 1994;11:519–532.
Viswanathan S, Frishman LJ, Robson JG, et al. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 1999;40:1124–1136.
Horiguchi M, Suzuki S, Kondo M, et al. Effect of glutamate analogues and inhibitory neurotransmitters on the electroretinograms elicited by random sequence stimuli in rabbits. Invest Ophthalmol Vis Sci 1998;39:2171–2176.
Gjörloff KW, Andréasson S, Ehinger B. Standardized full-field and multifocal electroretinography in rabbits. Doc Ophthalmol 2004;109:163–168.
Gjörloff KW, Andréasson S, Ghosh F. mfERG in normal and lesioned rabbit retina. Graefes Arch Clin Exp Ophthalmol 2006;244:83–89.
Gjörloff KW, Malmsjö M, Andréasson S, et al. Retinal function and PKC alpha expression after focal laser photocoagulation. Graefes Arch Clin Exp Ophthalmol 2007;245:1815–1824.
Gjörloff KW, Andréasson S, Ghosh F. Retinal function after vitrectomy. Retina 2008;28:558–563.
Hayasaka A, Machida S, Ishibe T, et al. Change of the full-field cone and multifocal ERGs after retinal peripapillary cauterization in cats. J Iwate Med Assoc 2005;57:367–382.
Ng YF, Chan HH, Chu PH, et al. Pharmacologically defined components of the normal porcine multifocal ERG. Doc Ophthalmol 2008;116;165–176.
Famiglietti EV, Sharpe SJ. Regional topography of rod and immunocytochemically characterized “blue” and “green” cone photoreceptors in rabbit retina. Vis Neurosci 1995;12:1151–1175.
Cideciyan AV, Hood DC, Huang Y, et al. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc Natl Acad Sci U S A 1998;95:7103–7108.
Machida S, Kondo M, Jamison JA, et al. P23H rhodopsin transgenic rat: correlation of retinal function with histopathology. Invest Ophthalmol Vis Sci 2000;41:3200–3209.
Aleman TS, LaVail MM, Montemayor R, et al. Augmented rod bipolar cell function in partial receptor loss: an ERG study in P23H rhodopsin transgenic and aging normal rats. Vision Res 2001;21:2779–2797.
Bush RA, Hawks KW, Sieving PA. Preservation of inner retinal responses in the aged Royal College of Surgeons rat. Evidence against glutamate excitotoxicity in photoreceptor degeneration. Invest Ophthalmol Vis Sci 1995;36:2054–2062.
Ohzeki T, Machida S, Takahashi T, et al. The effect of intravitreal N-methyl-dl-aspartic acid on the electroretinogram in Royal College of Surgeons rats. Jpn J Ophthalmol 2007;51:165–174.
Machida S, Raz-Prag D, Fariss RN, et al. Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci 2008;49:442–452.
Banin E, Cideciyan AV, Aleman TS, et al. Retinal rod photoreceptor-specific gene mutation perturbs cone pathway development. Neuron 1999;23:549–557.
Peng YW, Senda T, Hao Y, et al. Ectopic synaptogenesis during degeneration in the royal college of surgeons rat. Neuroscience 2003;119:813–820.
Haverkamp S, Michalakis S, Claes E, et al. Synaptic plasticity in CNG3(−/−) mice: cone bipolar cell react on the missing cone input and form ectopic synapses with rods. J Neurosci 2006;26:5248–5255.
Strettoi E, Mears AJ, Swaroop A. Recruitment of the rod pathway by cones in the absence of rods. J Neurosci 2004;24:7576–7582.
Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet 2001;29:447–452.
Jaissle GB, May CA, Reinhard J, et al. Evaluation of the rhodopsin knockout mouse as a model of pure cone function. Invest Ophthalmol Vis Sci 2001;42:506–513.
Jansen HG, Sanyal S. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol 1984;224:71–84.
Jansen HG, Sanyal S. Synaptic changes in the terminals of rod photoreceptors of albino mice after partial visual cell loss induced by brief exposure to constant light. Cell Tissue Res 1987;250:43–52.
Jansen HG, Sanyal S. Synaptic plasticity in the rod terminals after partial photoreceptor cell loss in the heterozygous rds mutant mouse. J Comp Neurol 1992;316:117–125.
Marc RE, Jones BW, Anderson JR, et al. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci 2007;48:3364–3371.
Grunder T, Kohler K, Guether E. Alternations in NMDA receptor expression during retinal degeneration in the RCS rat. Vis Neurosci 2001;18:781–787.
Kraft TW, Allen D, Petters RM, et al. Altered light responses of single rod photoreceptors in transgenic pigs expressing P347L or P347S rhodopsin. Mol Vis 2005;31:1246–1256.
Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci 1986;6:3505–3517.
Shimada Y, Li Y, Bearse MA, et al. Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol 2001;85:414–419.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yokoyama, D., Machida, S., Kondo, M. et al. Pharmacological dissection of multifocal electroretinograms of rabbits with Pro347Leu rhodopsin mutation. Jpn J Ophthalmol 54, 458–466 (2010). https://doi.org/10.1007/s10384-010-0842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-010-0842-9