Summary
Background
This study aimed to evaluate plasma relaxin‑2 (RLN-2) levels in patients with arterial hypertension (AH) and their relationships with clinical and laboratory parameters.
Methods
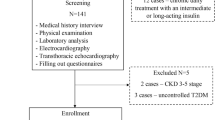
The study involved 106 hypertensive patients, including 55 with type 2 diabetes mellitus (T2DM), and 30 control subjects. Plasma RLN-2 levels were measured using an enzyme-linked immunosorbent assay kit.
Results
RLN-2 levels were reduced in patients with AH compared to healthy volunteers (p < 0.001), and hypertensive patients with T2DM had lower RLN-2 levels than those without impaired glucose metabolism (p < 0.001). RLN‑2 was negatively correlated with systolic blood pressure (SBP) (p < 0.001) and anthropometric parameters such as body mass index (BMI; p = 0.027), neck (p = 0.045) and waist (p = 0.003) circumferences, and waist-to-hip ratio (p = 0.011). RLN‑2 also had inverse associations with uric acid levels (p = 0.019) and lipid profile parameters, particularly triglycerides (p < 0.001) and non-HDL-C/HDL‑C (p < 0.001), and a positive relationship with HDL‑C (p < 0.001). RLN‑2 was negatively associated with glucose (p < 0.001), insulin (p = 0.043), HbA1c (p < 0.001), and HOMA-IR index (p < 0.001). Univariate binary logistic regression identified RLN‑2 as a significant predictor of impaired glucose metabolism (p < 0.001).
Conclusions
Decreased RLN-2 levels in patients with AH and T2DM and established relationships of RLN‑2 with SBP and parameters of glucose metabolism and lipid profile suggest a diagnostic role of RLN‑2 as a biomarker for AH with T2DM.
Zusammenfassung
Hintergrund
Ziel der vorliegenden Studie war es, die Konzentrationen von Plasma-Relaxin‑2 (RLN-2) bei Patienten mit arterieller Hypertonie (AH) und ihre Beziehung zu klinischen und labormedizinischen Parametern zu untersuchen.
Methoden
An der Studie nahmen 106 AH-Patienten teil, davon 55 mit Diabetes mellitus Typ 2 (Typ-2-Diabetes) und 30 Kontrollpersonen. Die Plasmaspiegel von RLN‑2 wurden mit einem Enzymimmunoassay gemessen.
Ergebnisse
Die RLN-2-Werte waren bei AH-Patienten im Vergleich zu gesunden Probanden erniedrigt (p < 0,001), und AH-Patienten mit Typ-2-Diabetes wiesen niedrigere RLN-2-Werte auf als Patienten ohne gestörten Glukosestoffwechsel (p < 0,001). RLN‑2 war negativ korreliert mit dem systolischen Blutdruck (SBP; p < 0,001) und mit anthropometrischen Parametern wie Body-Mass-Index (BMI; p = 0,027), Halsumfang (p = 0,045), Taillenumfang (p = 0,003) und Taille-Hüfte-Verhältnis (p = 0,011). Für RLN‑2 bestand auch ein inverser Zusammenhang mit dem Harnsäurespiegel (p = 0,019) und den Lipidprofilparametern, insbesondere mit Triglyzeriden (p < 0,001) und Nicht-High-Density-Lipoprotein-Cholesterin (Non-HDL-C)/HDL‑C (p < 0,001), außerdem lag eine positive Korrelation mit HDL‑C vor (p < 0,001). RLN‑2 war negativ mit Glukose (p < 0,001), Insulin (p = 0,043), HbA1c (p < 0,001) und dem HOMA-IR-Index (Homeostatic Model Assessment for Insulin Resistance; p < 0,001) assoziiert. In der univariaten binären logistischen Regression erwies sich RLN‑2 als signifikanter Prädiktor für einen gestörten Glukosestoffwechsel (p < 0,001).
Schlussfolgerung
Erniedrigte RLN-2-Werte bei Patienten mit AH und Typ-2-Diabetes und nachgewiesene Beziehungen zwischen RLN‑2 und SBP sowie Parametern des Glukosestoffwechsels und des Lipidprofils deuten auf eine diagnostische Rolle von RLN‑2 als Biomarker für AH mit Typ-2-Diabetes hin.



Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
World Health Organization (2020) Global Health Estimates 2019 Summary Tables: Deaths by Cause, Age And Sex, by World Bank Income Group, 2000–2019. https://www.who.int/docs/default-source/gho-documents/global-health-estimates/ghe2019_cod_wbincome_2000_201933383745-a750-4d94-8491-fb209dcece6f.xlsx?sfvrsn=e7bafa8_5. Accessed 09 September 2023.
Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41(6):389–93.
Bathgate RA, Halls ML, van der Westhuizen ET, et al. Relaxin family peptides and their receptors. Physiol Rev. 2013;93(1):405–80.
Dschietzig T, Richter C, Bartsch C, et al. The pregnancy hormone relaxin is a player in human heart failure. Faseb J. 2001;15(12):2187–95.
Aragón-Herrera A, Feijóo-Bandín S, Anido-Varela L, et al. Relaxin‑2 as a Potential Biomarker in Cardiovascular Diseases. J Pers Med. 2022;12(7):1021.
Feijóo-Bandín S, Aragón-Herrera A, Rodríguez-Penas D, et al. Relaxin‑2 in Cardiometabolic Diseases: Mechanisms of Action and Future Perspectives. Front Physiol. 2017;8:599.
Hossain MA, Praveen P, Noorzi NA, et al. Development of Novel High-Affinity Antagonists for the Relaxin Family Peptide Receptor 1. Acs Pharmacol Transl Sci. 2023;6(5):842–53.
Lian X, Beer-Hammer S, König GM, et al. RXFP1 Receptor Activation by Relaxin‑2 Induces Vascular Relaxation in Mice via a Gαi2-Protein/PI3Kß/γ/Nitric Oxide-Coupled Pathway. Front Physiol. 2018;9:1234.
Sasser JM, Cunningham MW Jr, Baylis C. Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Renal Physiol. 2014;307(12):F1355–F62.
Snowdon VK, Lachlan NJ, Hoy AM, et al. Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial. PLoS Med. 2017;14(2):e1002248.
Xu Q, Chakravorty A, Bathgate RA, Dart AM, Du XJ. Relaxin therapy reverses large artery remodeling and improves arterial compliance in senescent spontaneously hypertensive rats. Hypertension. 2010;55(5):1260–6.
Ponikowski P, Mitrovic V, Ruda M, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35(7):431–41.
Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin‑2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39.
Metra M, Teerlink JR, Cotter G, et al. Effects of Serelaxin in Patients with Acute Heart Failure. N Engl J Med. 2019;381(8):716–26.
Corcoran D, Radjenovic A, Mordi IR, et al. Vascular effects of serelaxin in patients with stable coronary artery disease: a randomized placebo-controlled trial. Cardiovasc Res. 2021;117(1):320–9.
Kobalava Z, Villevalde S, Kotovskaya Y, et al. Pharmacokinetics of serelaxin in patients with hepatic impairment: a single-dose, open-label, parallel group study. Br J Clin Pharmacol. 2015;79(6):937–45.
Gifford FJ, Dunne PDJ, Weir G, et al. A phase 2 randomised controlled trial of serelaxin to lower portal pressure in cirrhosis (STOPP). Trials. 2020;21(1):260.
Dahlke M, Halabi A, Canadi J, Tsubouchi C, Machineni S, Pang Y. Pharmacokinetics of serelaxin in patients with severe renal impairment or end-stage renal disease requiring hemodialysis: A single-dose, open-label, parallel-group study. J Clin Pharmacol. 2016;56(4):474–83.
Khanna D, Clements PJ, Furst DE, et al. Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(4):1102–11.
Sonaglia F, Milia P, Caserio M, et al. Efficacy and safety of oral porcine relaxin (pRLX) in adjunct to physical exercise in the treatment of peripheral arterial disease (PAD). Ital J Anat Embryol. 2013;118(1 Suppl):84–91.
Gedikli O, Yilmaz H, Kiris A, et al. Circulating levels of relaxin and its relation to cardiovascular function in patients with hypertension. Blood Press. 2009;18(1–2:68–73.
Zhang X, Zhu M, Zhao M, et al. The plasma levels of relaxin‑2 and relaxin‑3 in patients with diabetes. Clin Biochem. 2013;46(16–17:1713–6.
Szepietowska B, Gorska M, Szelachowska M. Plasma relaxin concentration is related to beta-cell function and insulin sensitivity in women with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;79(3):e1–e3.
Gao X, Li H, Wang P, Chen H. Decreased Serum Relaxin‑2 Is Correlated with Impaired Islet β‑Cell Function in Patients with Unstable Angina and Abnormal Glucose Metabolism. Int Heart J. 2018;59(2):272–8.
Bryant GD, Panter ME, Stelmasiak T. Immunoreactive relaxin in human serum during the menstrual cycle. J Clin Endocrinol Metab. 1975;41(06):1065–9.
Nose-Ogura S, Yoshino O, Yamada-Nomoto K, et al. Oral contraceptive therapy reduces serum relaxin‑2 in elite female athletes. J Obstet Gynaecol Res. 2017;43(3):530–5.
Casey E, Anderson T, Wideman L, et al. Optimal Paradigms for Measuring Peak Serum Relaxin in Eumenorrheic, Active Females. Reprod Med Int. 2018;1:6.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension [published correction appears in Eur Heart J. 2019 Feb 1;40(5):475]. Eur Heart J. 2018;39(33):3021–104.
American Diabetes Association Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin. Diabetes. 2022;40(1):10–38.
Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney. Dis Kidney Inter Suppl. 2013;3(1):1–150.
World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO. Ser, Vol. 854. Committee. World Health Organ Tech Rep: Expert; 1995. pp. 1–452.
World Health Organization. Noncommunicable Diseases and Mental Health Cluster (2020) WHO STEPS Surveillance Manual: the WHO STEPwise approach to noncommunicable disease risk factor surveillance. https://www.who.int/docs/default-source/ncds/ncd-surveillance/steps/steps-manual.pdf?sfvrsn=c281673d_5. Accessed 09 September 2023.
Bryant GD, Sassin JF, Weitzman ED, et al. Relaxin immunoactivity in human plasma during a 24-hr period. J Reprod Fertil. 1976;48(2):389–92.
Pintalhao M, Castro-Chaves P, Vasques-Novoa F, et al. Relaxin serum levels in acute heart failure are associated with pulmonary hypertension and right heart overload. Eur J Heart Fail. 2017;19(2):218–25.
Kupari M, Mikkola TS, Turto H, et al. Is the pregnancy hormone relaxin an important player in human heart failure? Eur J Heart Fail. 2005;7(2):195–8.
Aragón-Herrera A, Couselo-Seijas M, Feijóo-Bandín S, et al. Relaxin‑2 plasma levels in atrial fibrillation are linked to inflammation and oxidative stress markers. Sci Rep. 2022;12(1:22287.
Wolf JM, Cameron KL, Clifton KB, et al. Serum relaxin levels in young athletic men are comparable with those in women. Orthopedics. 2013;36(2):128–31.
Dragoo JL, Castillo TN, Braun HJ, et al. Prospective correlation between serum relaxin concentration and anterior cruciate ligament tears among elite collegiate female athletes. Am J Sports Med. 2011;39(10):2175–80.
Owens BD, Cameron KL, Clifton KB, et al. Association Between Serum Relaxin and Subsequent Shoulder Instability. Orthopedics. 2016;39(4):e724–e8.
Nonhoff J, Ricke-Hoch M, Mueller M, et al. Serelaxin treatment promotes adaptive hypertrophy but does not prevent heart failure in experimental peripartum cardiomyopathy. Cardiovasc Res. 2017;113(6):598–608.
Dragoo JL, Castillo TN, Korotkova TA, et al. Trends in serum relaxin concentration among elite collegiate female athletes. Int J Womens Health. 2011;3:19–24.
Giordano N, Papakostas P, Lucani B, et al. Serum relaxin in systemic sclerosis. J Rheumatol. 2005;32(11):2164–6.
Binder C, Simon A, Binder L, et al. Elevated concentrations of serum relaxin are associated with metastatic disease in breast cancer patients. Breast Cancer Res Treat. 2004;87(2):157–66.
Krüger S, Graf J, Merx MW, et al. Relaxin kinetics during dynamic exercise in patients with chronic heart failure. Eur J Intern Med. 2004;15(1):54–6.
Wolf JM, Scher DL, Etchill EW, et al. Relationship of relaxin hormone and thumb carpometacarpal joint arthritis. Clin Orthop Relat Res. 2014;472(4):1130–7.
Schöndorf T, Lübben G, Hoopmann M, et al. Relaxin expression correlates significantly with serum fibrinogen variation in response to antidiabetic treatment in women with type 2 diabetes mellitus. Gynecol Endocrinol. 2007;23(6):356–60.
Samuel CS, Royce SG, Hewitson TD, Denton KM, Cooney TE, Bennett RG. Anti-fibrotic actions of relaxin [published correction appears in Br J Pharmacol. 2017;174(24):4836]. Br J Pharmacol. 2017;174(10):962–976.
Wolf JM, Williams AE, Delaronde S, et al. Relationship of serum relaxin to generalized and trapezial-metacarpal joint laxity. J Hand Surg Am. 2013;38(4):721–8.
Papadopoulos DP, Makris T, Perrea D, et al. Apelin and relaxin plasma levels in young healthy offspring of patients with essential hypertension. J Clin Hypertens (greenwich). 2014;16(3):198–201.
Papadopoulos DP, Mourouzis I, Faselis C, et al. Masked hypertension and atherogenesis: the impact of apelin and relaxin plasma levels. J Clin Hypertens (greenwich). 2013;15(5):333–6.
Sanidas E, Tsakalis K, Papadopoulos DP, et al. The impact of apelin and relaxin plasma levels in masked hypertension and white coat hypertension. J Clin Hypertens (greenwich). 2019;21(1):48–52.
Bonanno A, Riccobono L, Bonsignore MR, et al. Relaxin in Obstructive Sleep Apnea: Relationship with Blood Pressure and Inflammatory Mediators. Respiration. 2016;91(1):56–62.
Lopez AY, Dereke J, Landin-Olsson M, et al. Plasma levels of relaxin‑2 are higher and correlated to C‑peptide levels in early gestational diabetes mellitus. Endocrine. 2017;57(3):545–7.
Wang P, Li HW, Wang YP, Chen H, Zhang P. Effects of recombinant human relaxin upon proliferation of cardiac fibroblast and synthesis of collagen under high glucose condition. J Endocrinol Invest. 2009;32(3):242–7.
Bonner JS, Lantier L, Hocking KM, et al. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes. 2013;62(9):3251–60.
Bitto A, Irrera N, Minutoli L, et al. Relaxin improves multiple markers of wound healing and ameliorates the disturbed healing pattern of genetically diabetic mice. Clin Sci (lond). 2013;125(12):575–85.
Singh S, Simpson RL, Bennett RG. Relaxin activates peroxisome proliferator-activated receptor γ (PPARγ) through a pathway involving PPARγ coactivator 1α (PGC1α). J Biol Chem. 2015;290(2):950–9.
Ng HH, Leo CH, Parry LJ, Ritchie RH. Relaxin as a Therapeutic Target for the Cardiovascular Complications of Diabetes. Front Pharmacol. 2018;9:501.
Ng HH, Leo CH, Prakoso D, Qin C, Ritchie RH, Parry LJ. Serelaxin treatment reverses vascular dysfunction and left ventricular hypertrophy in a mouse model of Type 1 diabetes. Sci Rep. 2017;7:39604.
Dschietzig TB, Krause-Relle K, Hennequin M, et al. Relaxin‑2 does not ameliorate nephropathy in an experimental model of type‑1 diabetes. Kidney Blood Press Res. 2015;40(1):77–88.
Wong SE, Samuel CS, Kelly DJ, et al. The anti-fibrotic hormone relaxin is not reno-protective, despite being active, in an experimental model of type 1 diabetes. Protein Pept Lett. 2013;20(9):1029–38.
Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, Assi HI. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 2022;23(2):786.
Aragón-Herrera A, Feijóo-Bandín S, Abella V, et al. Serelaxin (recombinant human relaxin-2) treatment affects the endogenous synthesis of long chain poly-unsaturated fatty acids and induces substantial alterations of lipidome and metabolome profiles in rat cardiac tissue. Pharmacol Res. 2019;144:51–65.
Aragón-Herrera A, Feijóo-Bandín S, Moraña-Fernández S, et al. Relaxin has beneficial effects on liver lipidome and metabolic enzymes. Faseb J. 2021;35(7):e21737.
McGowan BM, Minnion JS, Murphy KG, et al. Central and peripheral administration of human relaxin‑2 to adult male rats inhibits food intake. Diabetes Obes Metab. 2010;12(12):1090–6.
Borghi C, Cicero AFG. Serum Uric Acid and Cardiometabolic Disease: Another Brick in the Wall? Hypertension. 2017;69(6:1011–3.
Author information
Authors and Affiliations
Contributions
O. Pankova: conceptualization, design, methodology, project administration, resource, data curation, investigation, formal analysis, visualization, validation, literature search, writing—original draft preparation. O. Korzh: conceptualization, design, methodology, validation, supervision, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
O. Pankova and O. Korzh declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The study protocol was approved by the Ethics Committee of the Medical-Sanitary Base of JSC “Kharkiv Tractor Plant” (date of approval: 21 September 2021).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pankova, O., Korzh, O. Significance of plasma relaxin-2 levels in patients with primary hypertension and type 2 diabetes mellitus. Wien Med Wochenschr (2024). https://doi.org/10.1007/s10354-024-01035-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10354-024-01035-x