Summary
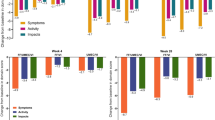
Health-related quality of life (HRQL) and patient-reported outcome (PRO) have become important outcome parameters for the evaluation of medical treatment within clinical trials and, furthermore, to evaluate efficiency in clinical practice. We therefore report further exploratory results of an already reported dose-finding study with EPs 7630 tablets, now focussing on HRQL and PRO. A total of 406 adults with acute bronchitis were randomly assigned to one of four parallel treatment groups (placebo, 30 mg, 60 mg or 90 mg EPs 7630 daily). HRQL and PRO were assessed by questionnaires as secondary outcome measures at each study visit or daily in the patient's diary. At day 7, the patient-reported outcome measures were significantly more improved in all the three EPs 7630 groups compared to placebo (EQ-5D and EQ VAS, SF-12: physical score, impact of patient's sickness, duration of activity limitation, patient-reported treatment outcome, satisfaction with treatment). In conclusion, a statistically significant and clinically relevant improvement of HRQL/PRO compared to placebo was shown in all the three EPs 7630 groups.
Zusammenfassung
Gesundheitsbezogene Lebensqualität (health-related quality of life, HRQL) und Therapiebeurteilung aus Patientensicht (patient-reported outcome, PRO) sind wichtige Erfolgsparameter in der Evaluierung einer medikamentösen Behandlung im Rahmen klinischer Studien und darüber hinaus zur Nutzenbewertung in der klinischen Praxis. Wir zeigen deshalb weitere, explorative Ergebnisse einer bereits berichteten Dosisfindungsstudie mit EPs 7630 Tabletten mit Fokus auf HRQL und PRO. Insgesamt wurden 406 Erwachsene mit akuter Bronchitis randomisiert einer von vier parallelen Behandlungsgruppen (Plazebo bzw. 30 mg, 60 mg oder 90 mg EPs 7630 täglich) zugeordnet. Mittels Patienten-Fragebögen wurden HRQL und PRO bei jeder Visite oder täglich im Tagebuch als sekundäre Zielparameter erhoben. An Tag 7 zeigte sich für alle drei EPs-7630-Gruppen im Vergleich zu Plazebo eine signifikante Verbesserung der durch die Patienten bewerteten Zielparameter (EQ-5D und EQ VAS, SF-12: physischer Komponentenscore, subjektives Krankheitsgefühl, Dauer der krankheitsbedingten physischen Beeinträchtigung, vom Patienten berichtetes Behandlungsergebnis, Therapiezufriedenheit). Insgesamt zeigte sich in allen drei EPs-7630-Gruppen im Vergleich zur Plazebo-Gruppe eine statistisch signifikante und klinisch relevante HRQL- sowie PRO-Verbesserung.
Similar content being viewed by others
Literatur
Johnson JR, Temple R. Food and Drug Administration requirements for approval of new anticancer drugs. Cancer Treat Rep, 69: 1155–1159, 1985
Garratt A, Schmidt L. Mackintosh A, et al. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ, 324: 1417–1419, 2002
Chassany O, Sagnier P, Marquis P, et al. Patient-reported outcomes. The example of health-related quality of life – a European guidance document for the improved integration of health-related quality of life assessment in the drug regulatory process. Drug Inform J, 36: 209–238, 2002
Revicki DA, Osoba D, Fairclough D, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res, 9: 887–900, 2000
Valderas JM, Alonso J. Patient reported outcome measures: a model-based classification system for research and clinical practice. Qual Life Res, 17: 1125–1135, 2008
Wiklund I. Assessment of patient-reported outcomes in clinical trials: the example of health-related quality of life. Fundam Clin Pharmacol, 18: 351–363, 2004
Centre for Health Economics. EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy, 16: 199–208, 1990
Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care, 34: 220–233, 1996
SF-36.org. A community to measure health outcome using SF tools. Available via http://www.sf-36.org/. Cited 10 May 2010
Schmier JK, Halpern MT, Mitchell K, et al. The quality of life impact of acute exacerbations of chronic bronchitis (AECB): a literature review. Qual Life Res, 14: 329–347, 2005
Doll H, Grey-Amante P, Duprat-Lomon I, et al. Quality of life in acute exacerbations of chronic bronchitis: results from a German population study. Respir Med, 96: 39–51, 2002
Cazzola M, MacNee W, Martinez FJ. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J, 31: 416–468, 2008
Chen H, Katz PP, Shiboski S, et al. Evaluating change in health-related quality of life in adult rhinitis: responsiveness of the Rhinosinusitis Disability Index. Health Qual Life Outcomes, 3: 68–78, 2005
Linder JA, Singer DE. Health-related quality of life of adults with upper respiratory tract infections. J Gen Intern Med, 18: 802–807, 2003
Aoki FY, Fleming DM, Griffin AD, et al. Impact of zanamivir treatment on productivity, health status and healthcare resource use in patients with influenza. Zanamivir Study Group. Pharmacoeconomics, 17: 187–195, 2000
Verheij T, Hermans J, Kaptein A, et al. Acute bronchitis: course of symptoms and restrictions in patients' daily activities. Scand J Prim Health Care, 13: 8–12, 1995
Kamin W, Maydannik VG, Malek FA, et al. Efficacy and tolerability of EPs 7630 in patients (aged 6–18 years old) with acute bronchitis: a randomized, double-blind, placebo-controlled clinical dose-finding study. Acta Paediatr, 99: 537–543, 2010
Kamin W, Maydannik V, Malek FA, et al. Efficacy and tolerability of EPs 7630 in children and adolescents with acute bronchitis: a randomized, double-blind, placebo-controlled multicenter trial with a herbal drug preparation from Pelargonium sidoides roots. Int J Pharmacol Ther, 48: 184–191, 2010
Matthys H, Kamin W, Funk P, et al. Pelargonium sidoides preparation (EPs® 7630) in the treatment of acute bronchitis in adults and children. Phytomedicine, 14(Suppl VI): 69–73, 2007
Chuchalin AG, Berman B, Lehmacher W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs® 7630): a randomized, double-blind, placebo-controlled trial. Explore, 1: 437–445, 2005
Agbabiaka TB, Guo R, Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine, 15: 378–385, 2008
Matthys H, Lizogub VG, Malek FA, et al. Efficacy and tolerability of EPs 7630 tablets in patients with acute bronchitis: a randomised, double-blind, placebo-controlled dose-finding study with a herbal drug preparation from Pelargonium sidoides. Curr Med Res Opin, 26: 1413–1422, 2010
EQ-5D. A standardised instrument for use as a measure of health outcome. Available via http://www.euroqol.org/. Cited 10 May 2010
Kolodziej H, Kiderlen AF. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs® 7630. Phytomedicine, 14(Suppl VI): 18–26, 2007
Schapowal A, Heger M. EPs® 7630 Lösung (Umckaloabo®) bei Sinusitis. Z Phytother, 28: 58–65, 2007
Bachert C, Schapowal A, Funk P, et al. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: a randomized, double-blind, placebo-controlled trial. Rhinology, 47: 51–58, 2009
Lizogub VG, Riley DS, Heger H. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: a randomized, double blind, placebo-controlled clinical trial. Explore, 3: 573–584, 2007
Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes, 5: 70–77, 2007
van Stel HF, Buskens E. Comparison of the SF-6D and the EQ-5D in patients with coronary heart disease. Health Qual Life Outcomes, 4: 20–28, 2006
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthys, H., Lizogub, V., Funk, P. et al. Pelargonium sidoides bei akuter Bronchitis – Gesundheitsbezogene Lebensqualität und Therapiebeurteilung aus Patientensicht unter Behandlung mit EPs 7630. Wien Med Wochenschr 160, 564–570 (2010). https://doi.org/10.1007/s10354-010-0847-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10354-010-0847-5