Summary
Background
Seroma formation is the major morbidity nuisance after breast cancer surgery. It prolonges hospital stay and causes infections and wound healing problems. The aim of the study was to evaluate the impact of the vessel sealing device LigaSure™ on postoperative morbidity especially seroma formation.
Methods
Between 2011 and 2012, data were retrospectively evaluated from 59 women with breast cancer who underwent axillary dissection at a university hospital. The Clavien Dindo Classification (CDC) was used to assess the severity of postoperative complications. Surgery was performed with LigaSure™ in 23 patients (39.0 %) compared with monopolar electrocautery in the control group.
Results
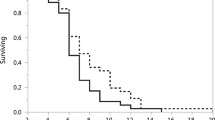
A total of 36 patients (61.0 %) had breast conserving surgery, and 5 patients (8.5 %) underwent isolated axillary dissection. Morbidity-associated re-operations were reported in 5.1 %. Seroma rates were nearly twice as high in the control group compared with LigaSure™ patients (p = 0.056). Hospital stay was shortened by 1 day using LigaSure™. The rate of punctures at the outpatient ward was half as high in the LigaSure™ cohort (21.7 vs. 41.7 %; p = 0.115). The frequency of aspirations (≤ 2 times) was lower in LigaSure™ patients. Despite this, the median puncture volume was higher (195 ml; IQR: 112.5–265 ml) in the LigaSure™ cohort as compared with the control group (100 ml; IQR: 60–180 ml). Overall, the CDC was significantly lower in the LigaSure™ group compared with the conventional cohort (p = 0.038).
Conclusions
The vessel sealing device LigaSure™ leads to a reduction of postoperative morbidity, hospital stay, and puncture frequency, which is desirable in terms of cost-effectiveness.


Similar content being viewed by others
References
Panhofer P, Ferenc V, Schütz M, et al. Standardization of morbidity assessment in breast cancer surgery using the Clavien Dindo Classification. Int J Surg. 2014;12(4):334–9. doi:10.1016/j.ijsu.2014.01.012.
Cortadellas T, Córdoba O, Espinosa-Bravo M, et al. Electrothermal bipolar vessel sealing system in axillary dissection: a prospective randomized clinical study. Int J Surg. 2011;9(8):636–40. doi:10.1016/j.ijsu.2011.08.002.
Van Bemmel AJ, van de Velde CJ, Schmitz RF, Liefers GJ. Prevention of seroma formation after axillary dissection in breast cancer: a systematic review. Eur J Surg Oncol. 2011;37(10):829–35. doi:10.1016/j.ejso.2011.04.012.
Galatius H, Okholm M, Hoffmann J. Mastectomy using ultrasonic dissection: effect on seroma formation. Breast. 2003;12(5):338–41.
Burak WE Jr., Goodman PS, Young DC, Farrar WB. Seroma formation following axillary dissection for breast cancer: risk factors and lack of influence of bovine thrombin. J Surg Oncol. 1997;64(1):27–31.
Schuijtvlot M, Sahu AK, Cawthorn SJ. A prospective audit of the use of a buttress suture to reduce seroma formation following axillary node dissection without drains. Breast. 2002;11(1):94–6.
Gong Y, Xu J, Shao J, et al. Prevention of seroma formation after mastectomy and axillary dissection by lymph vessel ligation and dead space closure: a randomized trial. Am J Surg. 2010;200(3):352–6. doi:10.1016/j.amjsurg.2009.10.013.
Carless PA, Henry DA. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br J Surg. 2006;93(7):810–9.
Moore M, Burak WE Jr., Nelson E, et al. Fibrin sealant reduces the duration and amount of fluid drainage after axillary dissection: a randomized prospective clinical trial. J Am Coll Surg. 2001;192(5):591–9.
Sanders RP, Goodman NC, Amiss LR Jr., et al. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. J Surg Res. 1996;61(1):65–70.
Warren HW, Griffith CD, McLean L, Angerson WJ, Kaye B, McElroy M. Should breast biopsy cavities be drained? Ann R Coll Surg Engl. 1994;76(1):39–41.
Zavotsky J, Jones RC, Brennan MB, Giuliano AE. Evaluation of axillary lymphadenectomy without axillary drainage for patients undergoing breast-conserving therapy. Ann Surg Oncol. 1998;5(3):227–31.
Somers RG, Jablon LK, Kaplan MJ, Sandler GL, Rosenblatt NK. The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer. A prospective randomized trial. Ann Surg. 1992;215(2):146–9.
Jansen RF, van Geel AN, de Groot HG, Rottier AB, Olthuis GA, van Putten WL. Immediate versus delayed shoulder exercises after axillary lymph node dissection. Am J Surg. 1990;160(5):481–4.
Rice DC, Morris SM, Sarr MG, et al. Intraoperative topical tetracycline sclerotherapy following mastectomy: a prospective, randomized trial. J Surg Oncol. 2000;73(4):224–7.
Carcoforo P, Soliani G, Maestroni U, et al. Octreotide in the treatment of lymphorrhea after axillary node dissection: a prospective randomized controlled trial. J Am Coll Surg. 2003;196(3):365–9.
Porter KA, O’Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. Am J Surg. 1998;176(1):8–11.
Keogh GW, Doughty JC, McArdle CSM, Cooke TG. Seroma formation related to electrocautery in breast surgery: a prospective randomized trial. Breast. 1998;7(1):39–41.
Hoefer RA Jr., DuBois JJ, Ostrow LB, Silver LF. Wound complications following modified radical mastectomy: an analysis of perioperative factors. J Am Osteopath Assoc. 1990;90(1):47–53.
Deo SV, Shukla NK. Modified radical mastectomy using harmonic scalpel. J Surg Oncol. 2000;74(3):204–7.
Lumachi F, Brandes AA, Burelli P, Basso SM, Iacobone M, Ermani M. Seroma prevention following axillary dissection in patients with breast cancer by using ultrasound scissors: a prospective clinical study. Eur J Surg Oncol. 2004;30(5):526–30.
Antonio M, Pietra T, Domenico L, et al. Does LigaSure reduce fluid drainage in axillary dissection? A randomized prospective clinical trial. Ecancermedicalscience. 2007;1:61. doi:10.3332/eCMS.2007.61.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Steger GG, Galid A, Gnant M, et al. Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG-14. J Clin Oncol. 2007;25(15):2012–8.
Steger GG, Greil R, Lang A, et al. Austrian Breast and Colorectal Study Group (ABCSG), Epirubicin and docetaxel with or without capecitabine as neoadjuvant treatment for early breast cancer: final results of a randomized phase III study (ABCSG-24). Ann Oncol. 2014;25(2):366–71. doi:10.1093/annonc/mdt508.
Morrow M. Margins in breast-conserving therapy: have we lost sight of the big picture? Expert Rev Anticancer Ther. 2008;8(8):1193–6. doi:10.1586/14737140.8.8.1193.
Fitzal F, Nehrer G, Hoch D, et al. An oncoplastic procedure for central and medio-cranial breast cancer. Eur J Surg Oncol. 2007;33(10):1158–63.
Puttawibul P, Sangthong B, Maipang T, Sampao S, Uttamakul P, Apakupakul N. Mastectomy without drain at pectoral area: a randomized controlled trial. J Med Assoc Thai. 2003;86(4):325–31.
Van Heurn LW, Brink PR. Prospective randomized trial of high versus low vacuum drainage after axillary lymphadenectomy. Br J Surg. 1995;82(7):931–2.
Droeser RA, Frey DM, Oertli D, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast. 2009;18(2):109–14. doi:10.1016/j.breast.2009.02.003.
Gupta R, Pate K, Varshney S, Goddard J, Royle GT. A comparison of 5-day and 8-day drainage following mastectomy and axillary clearance. Eur J Surg Oncol. 2001;27(1):26–30.
Lumachi F, Basso SM, Santeufemia DA, Bonamini M, Chiara GB. Ultrasonic dissection system technology in breast cancer: a case-control study in a large cohort of patients requiring axillary dissection. Breast Cancer Res Treat. 2013;142(2):399–404. doi:10.1007/s10549-013-2746-0.
Sanguinetti A, Docimo G, Ragusa M, et al. Ultrasound scissors versus electrocautery in axillary dissection: our experience. G Chir. 2010;31(4):151–3.
Ridings P, Bailey C, Bucknall TE. Argon beam coagulation as an adjunct in breast-conserving surgery. Ann R Coll Surg Engl. 1998;80(1):61–2.
Böhm D, Kubitza A, Lebrecht A, et al. Prospective randomized comparison of conventional instruments and the Harmonic Focus(®) device in breast-conserving therapy for primary breast cancer. Eur J Surg Oncol. 2012;38(2):118–24. doi:10.1016/j.ejso.2011.11.003.
Adwani A, Ebbs SR. Ultracision reduces acute blood loss but not seroma formation after mastectomy and axillary dissection: a pilot study. Int J Clin Pract. 2006;60(5):562–4.
Manouras A, Markogiannakis H, Genetzakis M, et al. Modified radical mastectomy with axillary dissection using the electrothermal bipolar vessel sealing system. Arch Surg. 2008;143(6):575–80. doi:10.1001/archsurg.143.6.575.
Lumachi F, Burelli P, Basso SM, Iacobone M, Ermani M. Usefulness of ultrasound scissors in reducing serous drainage after axillary dissection for breast cancer: a prospective randomized clinical study. Am Surg. 2004;70(1):80–4.
Lamberton GR, Hsi RS, Jin DH, Lindler TU, Jellison FC, Baldwin DD. Prospective comparison of four laparoscopic vessel ligation devices. J Endourol. 2008;22(10):2307–12. doi:10.1089/end.2008.9715.
Acknowledgments
The authors would like to thank Natalija Frank for carefully working on our database.
Conflict of interest
Peter Panhofer, Steffi Rothe, Michael Schütz, Barbara Grohmann-Izay, Peter Dubsky, Raimund Jakesz, and Michael Gnant have no personal or financial relationship to COVIDIEN MEDTRONIC. Florian Fitzal has had financial support from COVIDIEN concerning advanced training and science. Florian Fitzal has no financial support from COVIDIEN MEDTRONIC for the actual study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panhofer, P., Rothe, S., Schütz, M. et al. Morbidity reduction using the vessel sealing device LigaSure™ in breast cancer surgery. Eur Surg 47, 150–156 (2015). https://doi.org/10.1007/s10353-015-0334-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-015-0334-8