Abstract
Purpose
The Xeloda in Adjuvant Cancer Therapy trial, conducted in a white population of patients, established capecitabine (Xeloda) as adjuvant chemotherapy for Stage III colon cancer. Given the ethnical difference in toxicity of adjuvant chemotherapy in colon cancer, this study was designed to evaluate the safety and efficacy of adjuvant capecitabine in Chinese patients with colon cancer.
Methods
Chinese patients with curatively resected Stage III colon adenocarcinoma, who received adjuvant capecitabine, were entered into a prospective database. Oral capecitabine was given at 1,250 mg/m2 twice daily, Days 1 to 14, every 21 days, for 8 cycles. Toxicities, laboratory abnormalities, and survival outcomes were evaluated.
Results
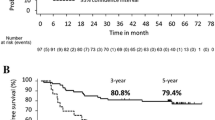
Fifty-eight patients were entered into the database between August 2004 and October 2005. The median age was 63.9 years with a male-to-female ratio of 1.15:1. With a median follow-up duration of 20.9 months, 14 patients relapsed and 3 patients died. Disease-free and overall survival at two years was 69 and 97 percent, respectively. Grade 3 toxicities occurred as follows: stomatitis (1.7 percent), diarrhea (0 percent), hand-foot syndrome (41.4 percent), leucopenia (1.7 percent), neutropenia (3.4 percent), and hyperbilirubinemia (1.7 percent). No Grade 4 or 5 toxicity was noted. Compared with the Xeloda in the Adjuvant Cancer Therapy trial, a much higher incidence of serious hand-foot syndrome and a lower rate of severe diarrhea were found in this study.
Conclusions
A different toxicity profile of adjuvant capecitabine was noted in this study on Chinese patients with colon cancer compared with that reported in the Xeloda in Adjuvant Cancer Therapy trial, whereas the efficacy outcomes were comparable.
Similar content being viewed by others
References
GLOBOCAN 2000: cancer incidence, mortality and prevalence worldwide, version 1.0. IARC CancerBase no. 5. Lyon, France: IARC Press, 2001.
International Multicentre Pooled Analysis of Colon Cancer Trials Investigators (IMPACT). Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 1995;345:939–44
Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806
Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. New Engl J Med 1990;32:352–8.
Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995;122:321–6.
Benson AB, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408–19.
Chau I, Cunningham D. Adjuvant therapy in colon cancer–what, when and how? Ann Oncol 2006;17:1347–59
Andre T, Colin P, Louvet C, et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol 2003;21:2896–903.
Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol 2005;23:1819–25.
Chau I, Norman AR, Cunningham D, et al. A randomized comparison between 6 months bolus fluorouracil/leucovorin and 12 weeks protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005;16:549–57.
Chau I, Norman AR, Cunningham D, et al. Longitudinal quality of life adjusted survival in a randomized controlled trial comparing six months of bolus fluorouracil/leucovorin vs. twelve weeks of protracted venous infusion fluorouracil as adjuvant chemotherapy for colorectal cancer. Eur J Cancer 2005;41:1551–9
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004;22:2214–32.
Schuller J, Cassidy J, Dumont E, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 2000;45:291–7.
Twelves C on behalf of the Xeloda Colorectal Cancer Group. Capecitabine as first-line treatment in colorectal cancer. Pooled data from two large, phase III trials. Eur J Cancer 2002;38 15–20
Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001;19:2282–92.
Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patient with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 2001;19:4097–106
Ward SE, Kaltenthaler E, Cowan J, Marples M, Orr B, Seymour MT. The clinical and economic benefits of capecitabine and tegafur with uracil in metastatic colorectal cancer. Br J Cancer 2006;95:27–34.
Twelves C, Boyer M, Findlay M, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer 2001;37:597–604.
Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
Engstrom PF, Benson AB, Chen YJ, et al. NCCN Colon Cancer Panel (2006) NCCN colon cancer clinical practice guidelines. Version 2. Available at: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf. Accessed July 24, 2006.
National Institute for Clinical Excellence (2006). Capecitabine and oxaliplatin in the adjuvant treatment of stage III colon cancer. Available at: http://www.nice.org.uk/page.aspx?o=TA100guidance. Accessed December 12, 2006.
McCollum AD, Catalano PJ, Haller DG, et al. Outcomes and toxicity in African American and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst 2002;94:1160–7.
Scheithauer W, McKendrick J, Begbie S, et al. Oral capecitabine as an alternative to iv 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 2003;14:1735–43.
Cassidy J, Scheithauer W, McKendrick J, et al. Capecitabine vs. bolus 5-FU/leucovorin as adjuvant therapy for colon cancer (the X-ACT study): efficacy results of a phase III trial. Proceedings of the annual meeting of the American Society of Clinical Oncology. J Clin Oncol 2004;22:3509.
Van Custem E, Findlay M, Osterwalder B, et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol 2000;18:1337–45
Bjornsson TD, Wagner JA, Donahue SR, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol 2003;43:943–67
Dignam JJ, Ye Y, Colangelo L, et al. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J Clin Oncol 2003;21:413–20
Dignam JJ, Colangelo L, Tian W, et al. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst 1999;91:1933–40
Hill HA, Eley JW, Harlan LC, Greenberg RS, Barrett RJ, Chen VW. Racial differences in endometrial cancer survival: the black/white cancer survival study. Obstet Gynecol 1996;88:919–26.
Patel DA, Barnholtz-Sloan JS, Patel MK, Malone JM, Chuba PJ, Schwartz K. A population-based study of racial and ethnic differences in survival among women with invasive cervical cancer: analysis of Surveillance, Epidemiology, and End Results data. Gynecol Oncol 2005;97:550–8
Gadgeel SM, Kalemkerian GP. Racial differences in lung cancer. Cancer Metastasis Rev 2003;22:39–46.
Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 2005;97:439–48
Al-Othman MO, Morris CG, Logan HL, Hinerman RW, Amdur RJ, Mendenhall WM. Impact of race on outcome after definitive radiotherapy for squamous cell carcinoma of the head and neck. Cancer 2003;98:2467–72.
Ma B, Yeo W, Hui P, Ho WM, Johnson PJ. Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer – a retrospective review of Chinese patients and comparison with an historic Western series. Radiother Oncol 2002;62:185–9.
O’Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002;20:2812–23.
Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005;23:792–9.
Roche Laboratories. Xeloda product information. April 2006. Retrieved from http://www.rocheusa.com/products/xeloda/pi.pdf; Accessed 4 November, 2006.
Marse H, Van Cutsem E, Grothey A, Valverde S. Management of adverse events and other practical considerations in patients receiving capecitabine. Eur J Oncol Nursing 2004;8:S16–30
Innocenti F, Ratain MJ. Update on pharmacogenetics in cancer chemotherapy. Eur J Cancer 2002;38:639–44.
van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer 2004;40:939–50
Maring JG, van Kuilenburg AB, Haasjes J, et al. Reduced 5-FU clearance in a patient with low DPD activity due to heterozygosity for a mutant allele of the DPYD gene. Br J Cancer 2002;86:1023–33
Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 1988;81:47–51
Takai S, Fernandez-Salguero P, Kimura S, Gonzalez FJ, Yamada K. Assignment of the human dihydropyrimidine dehydrogenase gene (DPYD) to chromosome region 1p22 by fluorescence in situ hydridization. Genomics 1994;24:613–4.
Wei X, Elizondo G, Sapone A, et al. Characterization of the human dihydropyrimidine dehydrogenase gene. Genomics 1998;51:391–400.
Wei X, McLeod HL, McMurrough J, Gonzalez FJ, Fernandez-Salguero P. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest 1996;98:610–5.
van Kuilenburg AB, Vreken P, Beex LV, et al. Heterozygosity for a point mutation in an invariant splice donor site of dihydropyrimidine dehydrogenase and severe 5-fluorouracil related toxicity. Eur J Cancer 1997;33:2258–64
Kouwaki M, Hamajima N, Sumi S, et al. Identification of novel mutations in the dihydropyrimidine dehydrogenase gene in a Japanese patient with 5-fluorouracil toxicity. Clin Cancer Res 1998;4:2999–3004.
Raida M, Schwabe W, Hausler P, et al. Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5′-splice donor site of intron 14 in patients with severe 5-fluorouracil-realted toxicity compared with controls. Clin Cancer Res 2001;7:2832–9
van Kuilenburg AB, Muller EW, Haasjes J, et al. Lethal outcome of a patient with a complete dihydropyrimidine dehydrogenase (DPD) deficiency after administration of 5-fluorouracil: frequency of the common IVS14 + 1G>A mutation causing DPD deficiency. Clin Cancer Res 2001;7:1149–53
Celik I, Kars A, Guc D, Tekuzman G, Ruacan S. Dihydropyrimidine dehydrogenase enzyme deficiency: clinical and genetic assessment of prevalence in Turkish cancer patients. Cancer Invest 2002;20:333–9
Yamaguchi K, Arai Y, Kanda Y, Akagi K. Germline mutation of dihydropyrimidine dehydrogenase gene among a Japanese population in relation to toxicity to 5-fluorouracil. Jpn J Cancer Res 2001;92:337–42.
Pullarkat ST, Stoehlmacher J, Ghaderi V, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J 2001;1:65–70.
van Kuilenburg AB, Meinsma JR, Zonnenberg BA, et al. Dihydropyrimidinase deficiency and severe 5-fluorouracil toxicity. Clin Cancer Res 2003;9:4363–7
Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Practice 2006;12:131–41.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Law, CC., Fu, YT., Chau, KK. et al. Toxicity Profile and Efficacy of Oral Capecitabine as Adjuvant Chemotherapy for Chinese Patients with Stage III Colon Cancer. Dis Colon Rectum 50, 2180–2187 (2007). https://doi.org/10.1007/s10350-007-9045-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10350-007-9045-y