Abstract
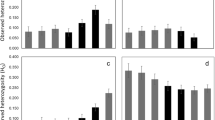
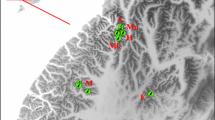
Wildlife conservation and management of endangered species requires reliable information on the size and structure of populations. One of the flagship species in European wildlife conservation is the forest-dwelling capercaillie (Tetrao urogallus), where several populations are endangered. In the Bohemian Forest, e.g., the population severely declined 30 years ago with only 100 birds remaining in 1985. Subsequently, breeding and release programs were conducted to supplement the local population. The current distribution and population size, however, remained unknown. With recent habitat changes and increasing recreational activities, a reliable population estimate to inform conservation plans was needed. A team of scientists and volunteers collected fresh capercaillie droppings covering an area of about 120,000 ha. We genotyped ten microsatellite loci to estimate the current population size and to determine the population’s spatial and genetic structure. Population size and density estimators revealed a population size of approximately 500 individuals, which is thus one of the two largest relict populations in the low mountain ranges of temperate Europe. The population clustering revealed gene flow across the entire study area. Several genotypes were documented with multiple recaptures at spatial distances between 10 and 30 km additionally corroborating gene flow across the entire landscape of the study area. Males were more closely related than females on small spatial scales up to 3 km, indicating lower dispersal rates in males. We conclude that the population currently appears to have a viable size and shows unrestricted gene flow across state borders and management units of the entire Bohemian Forest. However, long-term viability of this population requires a transboundary strategy to sustainably protect and monitor this isolated capercaillie population in Central Europe.




Similar content being viewed by others
References
Alda F, Sastre P, De La Cruz-Cardiel PJ, Doadrio I (2011) Population genetics of the endangered Cantabrian capercaillie in Northern Spain. Anim Cons 14:249–260. doi:10.1111/j.1469-1795.2010.00425.x
Alda F, González MA, Olea PP, Ena V, Godinho R, Drovetski SV (2013) Genetic diversity, structure and conservation of the endangered Cantabrian Capercaillie in a unique peripheral habitat. Eur J Wildl Res. doi:10.1007/s10344-013-0727-6
Arlettaz R, Patthey P, Baltic P, Leu T, Schaub M, Palme R, Jenni-Eiermann S (2007) Spreading free-riding snow sports represent a novel serious threat for wildlife. Proc R Soc Lond B 274:1219–1224. doi:10.1098/rspb.2006.0434
Baines D, Moss R, Dugan D (2004) Capercaillie breeding success in relation to forest habitat and predator abundance. J Appl Ecol 41:59–71. doi:10.1111/j.1365-2664.2004.00875.x
Bässler C (2004) Das Klima im Nationalpark Bayerischer Wald – Darstellung, Entwicklung und Auswirkung. Nationalparkverwaltung Bayerischer Wald, Grafenau, Germany
Beja-Pereira A, Oliveira R, Alves PC, Schwartz MK, Luikart G (2009) Advancing ecological understandings through technological transformations in noninvasive genetics. Mol Ecol Resour 9:1279–1301. doi:10.1111/j.1755-0998.2009.02699.x
BirdLife International (2012) Tetrao urogallus. In: IUCN 2013. IUCN red list of threatened species. Version 2013.2. www.iucnredlist.org. Downloaded on 29 March 2012
Bonin A, Nicole F, Pompanon F, Miaud C, Taberlet P (2007) Population adaptive index: a new method to help measure intraspecific genetic diversity and prioritize populations for conservation. Cons Biol 21:697–708. doi:10.1111/j.1523-1739.2007.00685.x
Bonter DN, Cooper CB (2012) Data validation in citizen science: a case study from project feederwatch. Front Ecol Environ 10:305–307. doi:10.1890/110273
Bouzat JL, Johnson K (2004) Genetic structure among closely spaced leks in a peripheral population of lesser prairie-chickens. Mol Ecol 13:499–505. doi:10.1046/j.1365-294X.2003.02068.x
Boyce MS (1992) Population viability analysis. Annu Rev Ecol Syst 23:481--506
Braunisch V, Segelbacher G, Hirzel AH (2010) Modelling functional landscape connectivity from genetic population structure: A new spatially explicit approach. Mol Ecol 19:3664--3678
Bufka L (2011) Verbreitung und Populationsentwicklung im Böhmerwald (Šumava). In: Das Auerhuhn im Oberen Bayerischen Wald und Böhmerwald (ed: Naturpark Oberer Bayerischer Wald) pp: 119–131
Burnham KP, Overton WS (1978) Estimation of the size of a closed population when capture probabilities vary among animals. Biometrika 65:625–633
Burnham KP, Overton WS (1979) Robust estimation of population size when capture probabilities vary among animals. Ecology 60:927–936
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A, Bunge J (2002) Estimating the number of species in a stochastic abundance model. Biometrics 58:531–539. doi:10.1111/j.0006-341X.2002.00531.x
Chao A, Lee SM (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87:210–217
Czech B, Krausman PR, Devers PK (2000) Economic associations among causes of species endangerment in the United States. BioScience 50:593–601. doi:10.1641/0006-3568(2000)050[0593:EAACOS]2.0.CO;2
Dickinson JL, Zuckerberg B, Bonter D (2010) Citizen science as an ecological research tool: challenges and benefits. Annu Rev Ecol Evol Syst 41:149–172. doi:10.1146/annurev-ecolsys-102209-144636
Efford MG (2011) Estimation of population density by spatially explicit capture-recapture analysis of data from area searches. Ecology 92:2202–2207
Efford MG, Fewster RM (2013) Estimating population size by spatially explicit capture-recapture. Oikos 122: 918--928
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611--2620
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567. doi:10.1111/j.1755-0998.2010.02847.x
Fedy BC, Martin K, Ritland C, Young J (2008) Genetic and ecological data provide incongruent interpretations of population structure and dispersal in naturally subdivided populations of white-tailed ptarmigan (Lagopus leucura). Mol Ecol 17:1905–1917. doi:10.1111/j.1365-294X.2008.03720.x
Fronhofer EA, Kubisch A, Hilker FM, Hovestadt T, Poethke HJ (2012) Why are metapopulations so rare? Ecology 93:1967–1978. doi:10.1890/11-1814.1
Gjerde I, Wegge P (1989) Spacing pattern, habitat use and survival of capercaillie in a fragmented winter habitat. Orn Scand 20:219–225
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. doi:10.1046/j.1365-294x.1998.00389.x
Grimm V, Storch I (2000) Minimum viable population size of capercaillie Tetrao urogallus: results from a stochastic model. Wildl Biol 6:219–225
Guillot G, Mortier F, Estoup A (2005) Geneland: a computer package for landscape genetics. Mol Ecol Notes 5:712–715. doi:10.1111/j.1471-8286.2005.01031.x
Guillot G, Renaud S, Ledevin R, Michaux J, Claude J (2012) A unifying model for the analysis of phenotypic, genetic, and geographic data. Syst Biol 61:897–911. doi:10.1093/sysbio/sys038
Hjorth I (1970) Reproductive behaviour in tetraonidae with special reference to males. Viltrevy 7:183--196
Hlavatá A (2002) Ekologie tetřeva hlušce (Tetrao urogallus). Diplomová práce, přírodovědecká fakulta UK Praha, m.s., 95 pp, http://www.npsumava.cz/cz/3501/4292/clanek/
Höglund J, Alatalo RV, Lundberg A, Rintamäki PT, Lindell J (1999) Microsatellite markers reveal the potential for kin selection on black grouse leks. Proc R Soc Lond B 266:813–816
Jacob G, Debrunner R, Gugerli F, Schmid B, Bollmann K (2010) Field surveys of capercaillie (Tetrao urogallus) in the Swiss Alps underestimated local abundance of the species as revealed by genetic analyses of non-invasive samples. Conserv Genet 11:1–12. doi:10.1007/s10592-008-9794-8
Johnson PCD, Haydon DT (2007) Maximum likelihood estimation of allelic dropout and false allele error rates from microsatellite genotypes in the absence of reference data. Genetics 175:827–842
Klaus S, Andreev AV, Bergmann HH, Müller F, Porkert J, Wiesner J (1989) Die Auerhühner. Neue Brehm Bücherei, Ziemsen Verlag, Wittenberg
Lacy RC, Sherman PW (1983) Kin recognition by phenotype matching. Am Nat 121:489–512
Lepczyk CA, Boyle OD, Vargo TL, Gould P, Jordan R, Liebenberg L, Masi S, Mueller WP, Prysby MD, Vaughan H (2009) Symposium 18: citizen science in ecology: the intersection of research and education. Bull Ecol Soc Am 90:308–317. doi:10.1890/0012-9623-90.3.308
Luikart G, Ryman N, Tallmon DA, Schwartz MK, Allendorf FW (2010) Estimation of census and effective population sizes: the increasing usefulness of DNA-based approaches. Conserv Genet 11:355–373
Lukacs PM, Burnham KP (2005) Review of capture-recapture methods applicable to noninvasive genetic sampling. Mol Ecol 14:3909–3919. doi:10.1111/j.1365-294X.2005.02717.x
Mäki-Petäys H, Corander J, Aalto J, Liukkonen T, Helle P, Orell M (2007) No genetic evidence of sex-biased dispersal in a lekking bird, the capercaillie (Tetrao urogallus). J Evol Biol 20:865–873. doi:10.1111/j.1420-9101.2007.01314.x
Marshall K, Edwards-Jones G (1998) Reintroducing capercaillie (Tetrao urogallus) into southern Scotland: identification of minimum viable populations at potential release sites. Biodiv Cons 7:275–296. doi:10.1023/A:1008844726747
Miller CR, Joyce P, Waits LP (2005) A new method for estimating the size of small populations from genetic mark-recapture data. Mol Ecol 14:1991–2005
Mollet P, Badilatti B, Bollmann K, Graf RF, Hess R, Jenny H, Mulhauser B, Perrenould A, Rudmann F, Sachot S (2003) Verbreitung und Bestand des Auerhuhns Tetrao urogallus in der Schweiz 2001 und ihre Veränderungen im 19. und 20. Jahrhundert. Ornithol Beob 100:67–86
Moss R, Picozzi N, Summers RW, Baines D (2000) Capercaillie Tetrao urogallus in Scotland—demography of a declining population. Ibis 142:259–267. doi:10.1111/j.1474-919X.2000.tb04865.x
Norris JL, Pollock KH (1998) Non-parametric MLE for Poisson species abundance models allowing for heterogeneity between species. Environ Ecol Stat 5:391–402. doi:10.1023/A:1009659922745
Paudel PK, Kindlmann P (2012) Human disturbance is a major determinant of wildlife distribution in Himalayan Midhill landscapes of Nepal. Anim Cons 15:283–293. doi:10.1111/j.1469-1795.2011.00514.x
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. doi:10.1111/j.1471-8286.2005.01155.x
Pennell MW, Stansbury CR, Waits LP, Miller CR (2013) Capwire: a R package for estimating population census size from non-invasive genetic sampling. Mol Ecol Resour 13:154–157
Piertney SB, Höglund J (2001) Polymorphic microsatellite DNA markers in black grouse (Tetrao tetrix). Mol Ecol Notes 1:303–304. doi:10.1046/j.1471-8278.2001.00118.x
Pollo CJ, Robles L, Sejas J, García-Miranda Á, Otero R (2003) Cantabrian capercaillie Tetrao urogallus cantabricus population size and range trend. Will the capercaillie survive in the Cantabrian mountains? Grouse News 26:3–5
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Quantum GIS Development Team (2012). Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project, http://qgis.osgeo.org
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, www.R-project.org
Ranta E, Lindstrom J, Linden H (1995) Synchrony in tetraonid population dynamics. J Animal Ecol 64:767–776
Regnaut S (2004) Population genetics of Capercaillie (Tetrao urogallus) in the Jura and the Pyrenees: a non-invasive approach to avian conservation genetics. Thesis at the Facultè de Biologie et de Mèdecine, Universite de Lausanne, France
Regnaut S, Lucas FS, Fumagalli L (2006) DNA degradation in avian faecal samples and feasibility of non-invasive genetic studies of threatened capercaillie populations. Conserv Genet 7:449–453. doi:10.1007/s10592-005-9023-7
Rolstad J (1989) Habitat and range use of capercaillie (Tetrao urogallus L.) in south central Scandinavian boreal forests, with spatial reference to the influence of modern forestry. PhD thesis, Department of Nature Conservation, Agricultural University, Norway
Rösner S, Mussard-Forster E, Lorenc T, Müller J (2014) Recreation shapes a “landscape of fear” for a threatened forest bird species in Central Europe. Landscape Ecol 29:55–66
Rutkowski R, Niewęgłowski H, Dziedzic R, Kmiec M, Godziewski J (2005) Genetic variability of the polish population of the capercaillie Tetrao urogallus. Acta Ornithol 40:27–34
Sachot S, Perrin N, Neet C (2006) Viability and management of an endangered Capercaillie (Tetrao urogallus) metapopulation in the Jura Mountains, Western Switzerland. Biod Cons 15:2017–2032
Saniga M (2002) Nest loss and chick mortality in capercaillie (Tetrao urogallus) and hazel grouse (Bonasa bonasia) in west Carpathians. Folia Zool 51:205–214
Scherzinger W (2003) Artenschutzprojekt Auerhuhn im Nationalpark Bayerischer Wald von 1985-2000. Nationalparkverwaltung Bayerischer Wald
Segelbacher G (2002) Noninvasive genetic analysis in birds: testing reliability of feather samples. Mol Ecol Notes. 2:367--369
Segelbacher G, Storch I (2002) Capercaillie in the Alps: genetic evidence of metapopulation structure and population decline. Mol Ecol 11:1669–1677
Segelbacher G, Piertney S (2007) Phylogeography of the European Capercaillie (Tetrao urogallus) and its implications for conservation. J Ornithol 148:269–274. doi:10.1007/s10336-007-0153-1
Segelbacher G, Paxton RJ, Steinbruck G, Trontelj P, Storch I (2000) Characterization of microsatellites in capercaillie Tetrao urogallus (AVES). Mol Ecol 9:1934--1935
Segelbacher G, Höglund J, Storch I (2003a) From connectivity to isolation: genetic consequences of population fragmentation in capercaillie across Europe. Mol Ecol 12:1773–1780
Segelbacher G, Storch I, Tomiuk J (2003b) Genetic evidence of capercaillie Tetrao urogallus dispersal sources and sinks in the Alps. Wild Biol 9:267–273
Segelbacher G, Wegge P, Sivkov AV, Höglund J (2007) Kin groups in closely spaced capercaillie leks. J Ornithol 148:79–84. doi:10.1007/s10336-006-0103-3
Segelbacher G, Manel S, Tomiuk J (2008) Temporal and spatial analyses disclose consequences of habitat fragmentation on the genetic diversity in capercaillie (Tetrao urogallus). Mol Ecol 17:2356–2367. doi:10.1111/j.1365-294X.2008.03767.x
Seidl R, Schelhaas M-J, Lexer MJ (2011) Unraveling the drivers of intensifying forest disturbance regimes in europe. Global Change Biology 17:2842--2852
Seiler C, Angelstam P, Bergmann H-H (2000) Conservation releases of captive-reared grouse in Europe. What do we know and what do we need? Cahiers d'Ethol 20:235–255
Smith DA, Ralls K, Hurt A, Adams B, Parker M, Maldonado JE (2006) Assessing reliability of microsatellite genotypes from kit fox faecal samples using genetic and GIS analyses. Mol Ecol 15:387–406. doi:10.1111/j.1365-294X.2005.02841.x
Smouse PE, Peakall R, Gonzales E (2008) A heterogeneity test for fine-scale genetic structure. Mol Ecol 17(14):3389–3400. doi:10.1111/j.1365-294X.2008.03839.x
Storch I (1993) Habitat use and spacing of capercaillie in relation to forest fragmentation patterns. PhD thesis, University of Munich, Germany
Storch I (1995) Habitat requirements of capercaillie. In: Jenkins D (ed) Proceedings of the 6th international symposium on grouse. World pheasant association, Great Britain, pp 151–154
Storch I (1997) Male territoriality, female range use, and spatial organisation of capercaillie Tetrao urogallus leks. Wildl Biol 3:149–161
Storch I (2001) Tetrao urogallus capercaillie, birds of Western Palaearctis. Update 3:1–24
Storch I (2007) Conservation status of grouse worldwide: an update. Wildl Biol 13:5–12
Storch I, Segelbacher G (2000) Genetic correlates of spatial population structure in central European capercaillie Tetrao urogallus and black grouse T. tetrix: a project in progress. Wildl Biol 6:305–310
Suchant R, Braunisch V (2004) Multidimensional habitat modelling in forest management: a case study using capercaillie in the black forest, Germany. Ecol Bull 51:455–469
Suchant R, Braunisch V (2008) Rahmenbedingungen und Handlungsfelder für den Aktionsplan Auerhuhn - Grundlagen für ein integratives Konzept zum Erhalt einer lebensfähigen Auerhuhnpopulation im Schwarzwald. - Forstliche Versuchs- und Forschungsanstalt Baden-Württemberg
Taylor AR, Knight RL (2003) Wildlife responses to recreation and associated visitor perceptions. Ecol Appl 13:951–963
Teuscher M, Brandl R, Rösner S, Bufka L, Lorenc T, Förster B, Hothorn T, Müller J (2011) Modelling habitat suitability for the Capercaillie Tetrao urogallus in the national parks Bavarian Forest and Šumava. Orn Anz 50:97–113
Teuscher M, Brandl R, Förster B, Hothorn T, Rösner S, Müller J (2013) Forest inventories are a valuable data source for habitat modelling of forest species: an alternative to remote-sensing data. Forestry 86:241–253. doi:10.1093/forestry/cps081
Thiel D, Jenni-Eiermann S, Palme R (2005) Measuring corticosterone metabolites in droppings of capercaillies (Tetrao urogallus). Ann N Y Acad Sci 1046:96–108. doi:10.1196/annals.1343.009
Thiel D, Ménoni E, Brenot JF, Jenni L (2007) Effects of recreation and hunting on flushing distance of capercaillie. J Wildl Manag 71:1784--1792
Thiel D, Jenni-Eiermann S, Jenni L (2008a) Der Einfluss von Freizeitaktivitäten auf das Fluchtverhalten, die Raumnutzung und die Stressphysiologie des Auerhuhns Tetrao urogallus. Orn Beob 105:85–96
Thiel D, Jenni-Eiermann S, Braunisch V, Palme R, Jenni L (2008b) Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: a new methodological approach. J Appl Ecol 45:845–853. doi:10.1111/j.1365-2664.2008.01465.x
Thiel D, Jenni-Eiermann S, Palme R, Jenni L (2011) Winter tourism increases stress hormone levels in the capercaillie Tetrao urogallus. Ibis 153:122--133
Thiollay JM, del Hoyo J, Elliott A, Sargatal J (1994) Handbook of the birds of the world. Vol. 2: new world vultures to guineafowl. Lynx Edicions, Barcelona
van der Zee D (1990) The complex relationship between landscape and recreation. Landscape Ecol 4:225–236
van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
von Blotzheim UG, Bauer KM, Bezzel E (1994) Handbuch der Vögel Mitteleuropas. Aula-Verlag, Wiesbaden
Wang JP (2010) Estimating species richness by a Poisson-compound gamma model. Biometrika 97:727–740. doi:10.1093/biomet/asq026
Wang JP (2011) SPECIES: an R package for species richness estimation. J Stat Software 40:1–15
Wang JPZ, Lindsay BG (2005). A penalized nonparametric maximum likelihood approach to species richness estimation. J Am Statist Assoc 100:942--959
Wang JPZ, Lindsay BG, Cui L, Wall PK, Marion J, Zhang J, dePamphilis CW (2005) Gene capture prediction and overlap estimation in EST sequencing from one or multiple libraries. BMC Bioinformat 6:300. doi:10.1186/1471-2105-6-300
Wegge P, Larsen BB (1987) Spacing of adult and subadult male common capercaillie during the breeding season. Auk 104:481–490
Wegge P, Rolstad J (1986) Size and spacing of capercaillie leks in relation to social behavior and habitat. Behav Ecol Sociobiol 19:401–408
Wegge P, Rolstad J (2011) Clearcutting forestry and Eurasian boreal forest grouse: long-term monitoring of sympatric capercaillie Tetrao urogallus and black grouse T. tetrix reveals unexpected effects on their population performances. Forest Ecol Manag 261:1520–1529. doi:10.1016/j.foreco.2011.01.041
Wilson GJ, Delahay RJ (2001) A review of methods to estimate the abundance of terrestrial carnivores using field signs and observation. Wildl Res 28:151–164. doi:10.1071/WR00033
Acknowledgments
We thank the rangers, foresters, hunters, and enthusiasts in Germany and the Czech Republic for their countless hours of fieldwork. We thank C. Budach, C. Heuck, G. Fischl, I. Möller, J. Macher, J. Stastny, E. Mussard-Forster, K.H. Schindlatz, K. Döringer, W. Scherzinger, M. Teuscher, N. & N. Farwig, S. Michl, and Y. Tiede for their general support, help during seminars, data collection, and working extensively in the field. We thank M. Teuscher for providing data. T.B. Mueller, D. Berens, and J. Albrecht helped with spatial and genetic analysis. T.B. Mueller helped with linguistic revision of the manuscript. We thank C. Scherer, S. Götzfried, and Y. Tiede for their help with laboratory work. Two anonymous referees and C. Gortázar helped to improve a previous version of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Rösner, S., Brandl, R., Segelbacher, G. et al. Noninvasive genetic sampling allows estimation of capercaillie numbers and population structure in the Bohemian Forest. Eur J Wildl Res 60, 789–801 (2014). https://doi.org/10.1007/s10344-014-0848-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-014-0848-6