Abstract
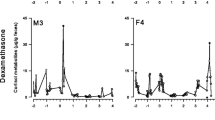
Rapid and reliable physiological evaluation of stress is necessary for understanding the potential impacts of environmental changes on managed populations of threatened mammals. In situ populations of Australia’s iconic marsupial, the greater bilby (Macrotis lagotis), are nearing extinction due to the impacts of competition and predation by feral animals and unpredictable climatic events (summer heat waves). In this study, we focussed our aim to identify a non-invasive method to measure adrenal activity in the species and also to identify potential factors that should be considered when comparing physiological stress in semi-free ranging populations of the species. We validated an enzyme immunoassay (EIA) for detecting fecal cortisol metabolites (FCM) from fresh fecal pellets taken from bilbies within four captive sites and two semi-free ranging populations around Queensland and New South Wales, Australia. Our FCM EIA successfully detected the ‘raise and fall’ pattern of FCM levels within 3 days of exogenous adrenocorticotropic hormone (ACTH) challenge. Mean FCM levels differed significantly between the captive sites and between sexes. All male bilbies grouped outdoor in captivity expressed the highest mean FCM level in comparison to all captive males that were housed individually or as groups indoors. Also, semi-free ranging bilbies expressed higher mean FCM levels than the captive bilbies. Overall, our study successfully validated a non-invasive tool for monitoring physiological stress in the greater bilby. In the future, it will be worthwhile to consider factors such as housing conditions, sex and location when comparing the adrenal sensitivity to environmental changes, to help evaluate the success of management interventions (such as predator free enclosures) and support the survival of the species.



Similar content being viewed by others
References
AWC (2012) Scotia Sanctuary. http://www.australianwildlife.org/AWC-Sanctuaries/Scotia-Sanctuary.aspx. Accessed 3/2/2012
Barja I, Silván G, Illera JC (2008) Relationships between sex and stress hormone levels in feces and marking behavior in a wild Population of Iberian wolves (Canis lupus signatus). J Chem Ecol 34(6):697–701
Benhaiem S, Dehnhard M, Bonanni R, Hofer H, Goymann W, Eulenberger K, East M (2012) Validation of an enzyme immunoassay for the measurement of faecal glucocorticoid metabolites in spotted hyenas (Crocuta crocuta). Gen Comp Endocrinol 178:265–271
Bonier F, Moore IT, Martin PR, Robertson RJ (2009) The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol 163(1–2):208–213
Bosacker AL (2008) Stress, evolution, and sociality in wild female baboons. 3310637. University of Minnesota, Minnesota
Buzzard PJ (2006) Cheek pouch use in relation to interspecific competition and predator risk for three guenon monkeys (Cercopithecus spp.). Primates 47(4):336–341
Cavigelli SA (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed femurs, Lemur catta. Anim Behav 57:935–944
Chapman CA, Saj TL, Snaith TV (2007) Temporal dynamics of nutrition, parasitism, and stress in Colobus monkeys: implications for population regulation and conservation. Am J Phys Anthropol 134:240–250
Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1(1):1--23
Creel S, Creel NM (1998) Six ecological factors that may limit African wild dogs, Lycaon pictus. Anim Conserv 1(1):1–9
Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S, Humphries MM, Boonstra R (2010) Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167(2):279–286
Dehnhard M, Clauss M, Lechner-Doll M, Meyer HHD, Palme R (2001) Noninvasive monitoring of adrenocortical activity in roe deer (Capreolus capreolus) by measurement of fecal cortisol metabolites. Gen Comp Endocrinol 123(1):111–120
DEHP (2012) Currawinya National Park. http://www.derm.qld.gov.au/parks/currawinya/index.html. Accessed 2/2/2012
Dunwoody E, Liu X, McDougall KA (2009) Spatial analysis of greater bilby (Macrotis lagotis) habitat in south-west queensland. In: Ostendorf B, Baldock P, Bruce D, Burdett M, Corcoran P (eds) Surveying & Spatial Sciences Institute Biennial International Conference. Spatial Sciences Institute, Adelaide, pp 977–986
Ellenberg U, Setiawan AN, Cree A, Houston DM, Seddon PJ (2007) Elevated hormonal stress response and reduced reproductive output in yellow-eyed penguins exposed to unregulated tourism. Gen Comp Endocrinol 152(1):54–63
Ellis T, James JD, Stewart C, Scott AP (2004) A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. J Fish Biol 65:1233–1252
Evans N, Narayan E, Hero J-M (2013) Effects of natural weathering conditions on glucocorticoid metabolite measurements in the greater bilby faeces. Aust J Zool 61:351–356
Fanson KV, Wielebnowski NC, Shenk TM, Lucas JR (2012) Comparative patterns of adrenal activity in captive and wild Canada lynx (Lynx canadensis). J Comp Physiol B Biochem Syst Environ Physiol 182(1):157–165
Finlayson GR, Vieira EM, Priddel D, Wheeler R, Bentley J, Dickman CR (2008) Multi-scale patterns of habitat use by re-introduced mammals: a case study using medium-sized marsupials. Biol Conserv 141(1):320–331
Forristal VE, Creel S, Taper ML, Scurlock BM, Cross PC (2012) Effects of supplemental feeding and aggregation on fecal glucocorticoid metabolite concentrations in elk. J Wildl Manag 76(4):694–702
Fuller G, Margulis SW, Santymire R (2011) The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo Biol 30(4):379–398
Gibson LA (2001) Seasonal changes in the diet, food availability and food preference of the greater bilby (Macrotis lagotis) in south-western Queensland. Wildl Res 28(2):121–134
Griffin B (2002) The use of fecal markers to facilitate sample collection in group-housed cats. Contemp Top Lab Anim Sci 41(2):51–56
Harper JM, Austad SN (2000) Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73(1):12–22
Hesterman H, Jones SM, Schwarzenberger F (2008) Reproductive endocrinology of the largest dasyurids: characterization of ovarian cycles by plasma and fecal steroid monitoring: Part I. The Tasmanian devil (Sarcophilus harrisii). Gen Comp Endocrinol 155(1):234–244
Hogan LA, Johnston SD, Lisle AT, Keeley T, Wong P, Nicolson V, Horsup AB, Janssen T, Phillips CJC (2011) Behavioural and physiological responses of captive wombats (Lasiorhinus latifrons) to regular handling by humans. Appl Anim Behav Sci 134(3):217–228
Hogan L, Lisle A, Johnston S, Robertson H (2013) Eliminative behaviour of captive numbats (Myrmecobius fasciatus): pattern and identification of faecal deposits. Zoo Biol, submitted for publication
IUCN (2006) IUCN Red List of Threatened Species.
James AI, Eldridge DJ (2007) Reintroduction of fossorial native mammals and potential impacts on ecosystem processes in an Australian desert landscape. Biol Conserv 138(3–4):351–359
Kalz B, Jewgenow K, Fickel J (2006) Structure of an otter (Lutra lutra) population in Germany — results of DNA and hormone analyses from faecal samples. Mamm Biol 71(6):321–335
Krebs CJ (1996) Population cycles revisited. J Mammal 77(1):8–24. doi:10.2307/1382705
Marechal L, Semple S, Majolo B, Qarro M, Heistermann M, MacLarnon A (2011) Impacts of tourism on anxiety and physiological stress levels in wild male Barbary macaques. Biol Conserv 144(9):2188–2193
McDonald IR, Lee AK, Bradley AJ, Than KA (1981) Endocrine changes in dasyurid marsupials with differing mortality patterns. Gen Comp Endocrinol 44(3):292–301
Millspaugh J, Washburn B (2004) Use of fecal glucocorticold metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138(3):189–199
Moseby KE, Hill BM, Read JL (2009) Arid recovery — a comparison of reptile and small mammal populations inside and outside a large rabbit, cat and fox-proof exclosure in arid South Australia. Aust Ecol 34(2):156–169
Muller MN, Wrangham RW (2004) Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55(4):332–340
Narayan E (2013) Non-invasive reproductive and stress endocrinology in amphibian conservation physiology. Conserv Physiol 1:1–16
Narayan E, Hero J-M (2014a) Acute thermal stressor increases glucocorticoid response but minimizes testosterone and locomotor performance in the cane toad (Rhinella marina). PLoS One 9:e92090
Narayan E, Hero J-M (2014b) Repeated thermal stressor causes chronic elevation of baseline corticosterone and suppresses the physiological endocrine sensitivity to acute stressor in the cane toad (Rhinella marina). J Therm Biol 41:72–76
Narayan E, Molinia F, Christi K, Morley C, Cockrem J (2010) Urinary corticosterone metabolite responses to capture and annual patterns of urinary corticosterone in wild and captive endangered Fijian ground frogs (Platymantis vitiana). Aust J Zool 58:189–197
Narayan E, Hero JM, Evans N, Nicolson V, Mucci A (2012) Non-invasive evaluation of physiological stress hormone responses in a captive population of the greater bilby Macrotis lagotis. Endanger Species Res 18:279–289
Narayan E, Cockrem JF, Hero J-M (2013a) Sight of a predator induces a corticosterone stress response and generates fear in an amphibian. PLoS One 8:e73564
Narayan E, Webster K, Nicolson V, Hero J-M (2013b) Non-invasive evaluation of physiological stress in an iconic Australian marsupial: the Koala (Phascolarctos cinereus). Gen Comp Endocrinol 187:39–47
Narayan E, Clark G, Martin-Vegue P, Parnell T, Mucci A, Hero J-M (2013c) Faecal cortisol metabolite levels in Bengal (Panthera tigris tigris) and Sumatran tigers (Panthera tigris sumatrae). Gen Comp Endocrinol 194:318–325
Owen MA, Swaisgood RR, Czekala NM, Steinman K, Lindburg DG (2004) Monitoring stress in captive giant pandas (Ailuropoda melanoleuca): behavioral and hormonal responses to ambient noise. Zoo Biol 23:147–164
Palme R (2005) Measuring fecal steroids — guidelines for practical application. In: Bauchinger U, Goymann W, JenniEiermann S (eds) Bird hormones and bird migrations: analyzing hormones in droppings and egg yolks and assessing adaptations in long-distance migration. Ann N Y Acad Sci 1046:75–80
Park HC, Han TY, Kim DC, Min MS, Han SY, Kim KS, Lee H (2011) Individual identification and sex determination of Eurasian otters (Lutra lutra) in Daegu city based on genetic analysis of otter spraint. Genes Genomics 33(6):653–657
Pavey C (2006) National recovery plan for the greater bilby, Macrotis lagotis. Northern Territory Department of Natural Resources, Environment and the Arts.
Rogovin K, Randall JA, Kolosova I, Moshkin M (2003) Social correlates of stress in adult males of the great gerbil, Rhombomys opimus, in years of high and low population densities. Horm Behav 43(1):132–139
Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Nino events. Proc Natl Acad Sci U S A 98(13):7366–7370
Schwarzenberger F (2007) The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int Zool Yearb 41(1):52–74
Sheriff MJ, Krebs CJ, Boonstra R (2009) The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J Anim Ecol 78(6):1249–1258
Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166:869–877
Smith S, McRae P, Hughes J (2009) Faecal DNA analysis enables genetic monitoring of the species recovery program for an arid-dwelling marsupial. Aust J Zool 57(2):139–148
Smith JE, Monclus R, Wantuck D, Florant GL, Blumstein DT (2012) Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen Comp Endocrinol 178(2):417–426
Stead-Richardson E, Bradshaw D, Friend T, Fletcher T (2010). Monitoring reproduction in the critically endangered marsupial, Gilbert’s potoroo (Potorous gilbertii): Preliminary analysis of faecal oestradiol-17 beta, cortisol and progestagens. Gen Comp Endocrinol 165(1):155--162
Terio KA, Marker L, Munson L (2004) Evidence for chronic stress in captive but not free-ranging cheetahs (Acinonyx jubatus) based on adrenal morphology and function. J Wildl Dis 40:259–266
Thaker M, Vanak AT, Lima SL, Hews DK (2010) Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor. Am Nat 175(1):50–60
Turner JW, Nemeth R, Rogers C (2003) Measurement of fecal glucocorticoids in parrotfishes to assess stress. Gen Comp Endocrinol 133:341–352
Wasser SK, Hunt KE (2005) Noninvasive measures of reproductive function and disturbance in the barred owl, great horned owl, and northern spotted owl. Ann N Y Acad 1046:109–113
Watts HE, Holekamp KE (2008) Interspecific competition influences reproduction in spotted hyenas. J Zool 276(4):402–410
Wielebnowski NC, Fletchall N, Carlstead K, Busso JM, Brown JL (2002) Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zool Biol 21(1):77–98
Wikelski M, Cooke SJ (2006) Conservation physiology. Trends Ecol Evol 21(1):38–46
Williams B, Manthey F (2012) Feral cats wreak havoc in raid on 'enclosed' refuge for endangered bilbies. The Courier-Mail, July 19. Online accessible.
Woodruff JA, Lacey EA, Bentley G (2010) Contrasting fecal corticosterone metabolite levels in captive and free-living colonial Tuco-tucos (Ctenomys sociabilis). J Exp Zool A 313A(8):498–507
Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL (2004) Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticold analyses. Gen Comp Endocrinol 137(2):148–165
Acknowledgments
This project was completed in accordance with approval from Griffith University’s Animal Ethics Committee (ENV/17/11/AEC). Field work was conducted under Queensland Department of Environment and Heritage Protection scientific permit number WITK10064911. This research was undertaken as an Honours Research Project by NE that was co-supervised jointly by EJN and J-MH. We would like to thank the Australian Wildlife Conservancy and Queensland National Parks and Wildlife Service for providing field equipment, on-site accommodation and other facilities whilst sampling in the field. Special thanks to numerous volunteers assisted with the fieldwork and Greg Lollback provided feedback on earlier version of this manuscript. We also thank the staff of Dreamworld, Ipswich Nature Centre, Currumbin Wildlife Sanctuary and Charleville Breeding centre for all their help and cooperation. We also thank the veterinarians Vere Nicolson and Michael Pyne who assisted with applying the ACTH and saline treatments. Funding was provided by Save the Bilby Fund and Griffith University. We are grateful to the editor and two anonymous referees for helpful reviews and comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. W. Sainsbury
Rights and permissions
About this article
Cite this article
Narayan, E.J., Evans, N. & Hero, JM. Monitoring physiological stress in semi-free ranging populations of an endangered Australian marsupial, the Greater Bilby (Macrotis lagotis). Eur J Wildl Res 60, 727–735 (2014). https://doi.org/10.1007/s10344-014-0842-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-014-0842-z