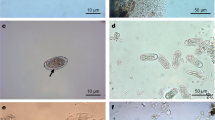
Abstract
Helminths have often been considered responsible in driving partially wildlife population density fluctuations; therefore more information have to be achieved when target hosts are endangered or threatened species, such as black grouse (Tetrao tetrix). During 8 years (2003–2010), we collected and analyzed 431 intestinal contents of hunted black grouse from Central Alps (Verbania), recording age, body weights, biometric measures, and the culling site to investigate (1) their helminth community structure, (2) how much infection varies in relation to age class and to prealpine and alpine origin area, and (3) if parasites may act as population destabilizing factors. The helminth community is composed by two nematodes Ascaridia compar and Aonchotheca caudinflata. A. compar is the predominant species, and a significant higher intensity of both helminths is recorded in juveniles. A. caudinflata has a negative effect on weights of all the population subjects (p < 0.001), independently of age and origin area. Prealpine population, characterized by significantly lower weights than those of the alpine ones (p < 0.05), is found more infected by both helminths, with also a negative impact (p < 0.001) of A. compar recorded on prealpine adults’ weights. The negative effect noticed for A. caudinflata emphasizes its pathogenicity and shows that this infection can be considered a further stressor for the studied population. Moreover, A. compar affects essentially adults in prealpine area and this fact, together with the major infection of the entire prealpine population, supports the hypothesis that habitat characteristics play a role in the infectious process.




Similar content being viewed by others
References
Anderson RC (1992) Nematode parasites of vertebrates: their development and transmission. CABI, Wallinford, pp 245–247, 545–546
Anderson RM, May RM (1979a) Population biology of infectious diseases. Part I. Nature 280:361–367
Anderson RM, May RM (1979b) Population biology of infectious diseases. Part II. Nature 280:455–461
Anfodillo T (2007) Cambiamenti climatici e dinamica di popolazione al limite superiore del bosco: importanza delle ricerche di lungo termine. Forest 4:3–5
Aschenbrenner R (1989) Parasite infections of wild and penned black grouse (Lyrurus tetrix) and capercaillie (Tetrao urogallus) in Austria. Dissertation, University of Veterinary Medicine, Vienna, pp. 139–157
Ashour AA (1994) Scanning electron microscopy of Ascaridia galli (Schrank, 1788), Freeborn, 1923 and A. columbae (Linstow, 1903). J Egypt Soc Parasitol 24:349–355
Barchetti A, De Marco MA, Guberti V (1999) Elminti gastrointestinali in tre specie di galliformi dell’arco alpino. La Selezione Veterinaria 8–9:699–704
Belleau E, Leonard P (1991) Le parasitisme digestif chez la perdiz bartavelle (Alectoris graeca saxatilis), la lagopede alpin (Lagopus mutus), le tètrao-lyre (Tetrao tetrix) dans le departement des Haute-Alpes. Gibier Faune Sauvage 8:161–173
Bowker G, Bowker C, Baines D (2007) Survival rates and causes of mortality in black grouse Tetrao tetrix at Lake Vyrnwy, North Wales, UK. Wildlife Biol 13:3
Brownell SA, Nelson KL (2006) Inactivation of single-celled Ascaris suum eggs by low-pressure UV radiation. Appl Environ Microb 3:2178–2184
Bush AO, Lafferty KD, Lotz JM, Shostack AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Citterio CV, Caslini C, Milani F, Sala M, Ferrari N, Lanfranchi P (2006) Abomasal nematode community in an alpine chamois (Rupicapra R. rupicapra) population before and after a die-off. J Parasitol 92:918–927
Delahay RJ (1999) Cestodiasis in the red grouse in Scotland. J Wildlife Dis 35:250–258
Development Core Team R (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Frosio GD, Sala M, Lanfranchi P, Gallazzi D (2000) Elmintofauna intestinale in galliformi autoctoni delle Alpi Orobie. Quadro epidemiologico e relative implicazioni gestionali (Intestinal helminths in free living galliformes in Orobie Alps and management implications). La Selezione Veterinaria 8–9:817–825
Gardner SG, Campbell ML (1992) Parasite as probes of biodiversity. J Parasitol 78:596–600
Gauly M, Bauer C, Preisinger R, Erhardt G (2002) Genetic differences of Ascaridia galli egg output in laying hens following a single dose infection. Vet Parasitol 103:99–107
Godfrey JD (2003) Potential use of energy expenditure of individual birds to assess quality of their habitats. In: Williams M (ed) Conservation applications of measuring energy expenditure of New Zealand birds: assessing habitat quality and costs of carrying radio transmitters. Department of Conservation, New Zealand, pp 11–24
Hartwich G (1978) Keys to genera of Ascaridoidea. Commonwealth Agricultural Bureaux, Oxfordshire, pp 1–5
Helminen M (1963) Composition of the Finnish populations of capercaillie, Tetrao urogallus, and black grouse, Lyrurus tetrix, in the autumns of 1952–1961, as revealed by a study of wings. Pap Game Res 23:1–124
Holmstad PR, Hudson PJ, Skorping A (2005) The influence of a parasite community on the dynamics of a host population: a longitudinal study on willow ptarmigan and their parasites. Oikos 111:377–391
Hudson PJ (1986) The effect of a parasitic nematode on the breeding production of red grouse. J Anim Ecol 55:85–92
Hudson PJ, Dobson AP (1995) Macroparasites: observed patterns in naturally fluctuating animal population. In: Grenfell BT, Dobson AP (eds) Ecology of infectious disease in natural population. Cambridge University Press, Cambridge, pp 146–176
Hudson PJ, Dobson AP, Newborn D (1992) Do parasites make prey vulnerable to predation? Red grouse and parasites. J Anim Ecol 61:681–692
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385. doi:10.1016/j
Isomursu M, Rätti O, Helle P, Hollmén T (2006) Sex and age influence intestinal parasite burden in three boreal grouse species. J Avian Biol 37:516–522
Isomursu M, Rätti O, Helle P, Hollmén T (2008) Parasitized grouse are more vulnerable to predation as revealed by a dog-assisted hunting study. Ann Zool Fenn 45:496–502
Istituto per le Piante da Legno e l’Ambiente - IPLA s.p.a. (2008) Valutazione e rilievi biometrici della fauna selvatica. Ungulati, galliformi alpini e lepre variabile. Osservatorio regionale sulla fauna selvatica. Regione Piemonte, pp 103–115
Jankovska I, Bejcek V, Langrova I, Válek P, Vadlejch J, Čadková Z (2012) Black grouse in Czech Republic and its parasites. Helminthologia 2:78–81. doi:10.2478/s11687-012-0016-z
Kalla PI, Dimmick RW, Patton S (1997) Helminths in ruffed grouse at the host’s southeastern range boundary. J Wildlife Dis 33:503–510
Kurki S, Helle P, Lindén H, Nikula A (1997) Breeding success of black grouse and capercaillie in relation to mammalian predator densities on two spatial scales. Oikos 79:301–310
Ludwig T, Storch I, Graf RF (2009) Historic landscape change and habitat loss: the case of black grouse in Lower Saxony, Germany. Landscape Ecol 4:533–546. doi:10.1007/s10980-357009-9330-3
Marcogliese DJ (2005) Parasites of the superorganism: are they indicators of ecosystem health? Int J Parasitol 35:705–716
Marcos-Atxutegi C, Gandolfi B, Arangüna T, Sepúlveda R, Arévalo M, Simón F (2009) Antibody and inflammatory responses in laying hens with experimental primary infections 361 of Ascaridia galli. Vet Parasitol 161:69–75. doi:10.1016/j
Mathews F (2009) Zoonoses in wildlife: integrating ecology into management. Adv Parasit 8:186–203
Mero WMS, Gazal ARD (2008) Effect of constant and changing temperatures on the development and viability of Ascaridia galli eggs. The 2nd Kurdistan conference on biological sciences. J Duhok Univ 12:35–38
Ministry of Agriculture, Fisheries and Food (MAFF) (1986) Manual of veterinary parasitological laboratory techniques. HMSO, London
Obeso JR, Rodríguez LD, Álvarez I, Eloy N, Del Campo JC (2000) Intestinal parasites in the Cantabrian capercaillie Tetrao urogallus cantabricus: a coprological study. Ardeola 47:191–195
Patthey P, Wirthner S, Signorell N, Arlettaz R (2008) Impact of outdoor winter sports on the abundance of a key indicator species of alpine ecosystems. J Appl Ecol 45:1704–1711
Pearce-Higgins JW, Grant MC, Robinson MC, Haysom SL (2007) The role of forest maturation in causing the decline of black grouse Tetrao tetrix. Ibis 149:143–155
Pinto RM, Tortelly R, Menezes RC, Gomes DC (2004) Trichurid nematodes in ring-necked pheasants from backyard flocks of the state of Rio de Janeiro, Brazil: frequency and pathology. Memórias do Instituto Oswaldo Cruz 99:721–726
Rizzoli AP, Rosso F, Ferrari N, Rosà R, Farrè L, Manfredi MT, Hudson PJ (2003) Infestazione da Ascaridia compar (Schrank, 1790) nella coturnice alpina: effetti sull’ovodeposizione e su alcuni valori ematochimici. Journal Mountain Ecology 7:291–294
Saunders LM, Tompkins DM, Hudson PJ (2000) The role of oxygen availability in the embryonation of Heterakis gallinarum eggs. Int J Parasitol 30:1481–1485
Schmidt-Hempel P, Koella JC (1994) Variability and its implications for host-parasite interactions. Parasitol Today 10:98–102
Signorell N, Wirthner S, Patthey P, Schranz R, Rotelli L, Arlettaz R (2010) Concealment from predators drives foraging habitat selection in brood-rearing Alpine black grouse Tetrao tetrix hens: habitat management implications. J Wildlife Biol 16:249–257. doi:40910.2981/09-028
Skrjabin KI, Shikhobalova NP, Orolov IV (1970) Tricocephalide and capillaride of animals and the disease caused by them. Israel program for scientific translation, Jerusalem, pp 297–299
Soulsby EJL (1987) Nematodes. In: Soulsby EJL (ed) Immune response in parasitic infection: immunology, immunopatology and immunophylaxis, vol 1. CRC, Boca Raton, pp 65–66
Storch I (2007) Grouse: status survey and conservation action plan 2006–2010. IUCN, Washington DC, pp 41–45
Taylor MA, Coop RL, Wall RL (2010) Parassitologia e Malattie Parassitarie degli Animali. Aggiornata e coordinata da Garippa G, Manfredi MT, Otranto D. Edizioni Mediche Scientifiche Internazionali (EMSI), p 10
Thieltges DW, Jensen KT, Poulin R (2008) The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135:407–426. doi:10.1017/S0031182007000248
Tinner W, Vescovi E (2007) Ecologia e oscillazioni del limite degli alberi nelle Alpi dal Pleniglaciale al presente. Studi Trentini Sci Nat Acta Geol 82:7–15
Watson A, Moss R (2004) Impacts of ski-development on ptarmigan (Lagopus mutus) at Cairn Gorm, Scotland. Biol Conserv 116:267–275
Wissler KE, Halvorsen O (1977) Helminths from willow grouse (Lagopus mutus) in two localities in North Norway. J Wildlife Dis 13:409–413
Acknowledgments
We are grateful to “ALCOTRA: Italy–France Alpine Grouse” Interreg Project that gave us the opportunity to carry out this research. We wish to thank all the hunters of the Alpine hunting territory in the province of Verbania for their help in the field activities and Ilaria Marangi for her invaluable suggestions that improved the drafting of this paper.
Ethical declaration
I, the undersigned, Nicoletta Formenti, declare that all the experiments used in the previous manuscript comply with the current Italian laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Formenti, N., Viganò, R., Rotelli, L. et al. Effect of suboptimal environment and host age on helminth community of black grouse (Tetrao tetrix). Eur J Wildl Res 59, 351–358 (2013). https://doi.org/10.1007/s10344-012-0681-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-012-0681-8