Abstract
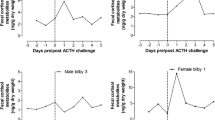
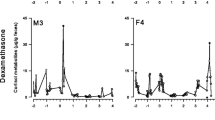
Measurement of faecal glucocorticoid metabolites is increasingly used as a non-invasive tool to examine disturbances in various domestic and wild animals. Because measurements of faecal glucocorticoid metabolites has previously never been reported in fallow deer, we determined 11,17-dioxoandrostanes (11,17-DOA), a group of cortisol metabolites, in the faeces of four fallow deer yearlings after an adrenocorticotropic hormone (ACTH) challenge or control saline injection by an 11-oxoaetiocholanolone enzyme immunoassay (EIA), to validate a method. A 2.9- to 4.3-fold increase in measured cortisol metabolites in challenged animals after approximately 22 h demonstrated the suitability of this group-specific EIA to monitor adrenocortical activity in respective deer species. To determine faecal cortisol metabolites in fallow deer from a Mediterranean habitat, we collected samples during a 1-year study at Veliki Brijuni Island. The study confirmed seasonal pattern of cortisol release in fallow deer. Higher 11,17-DOA concentrations (median; min–max) were determined for November (99; 50–2,035), March (112; 25–315) and May (92; 40–196 ng/g faeces). Significantly lower concentrations were measured during July (30; 10–195 ng/g faeces). This study indicates that the analysis of faecal glucocorticoid metabolites is a valuable non-invasive technique for monitoring adrenocortical activity in fallow deer. This, together with information about the seasonal pattern of glucocorticoid excretion, could help to improve fallow deer management and welfare, especially in the case of farmed and park animals.



Similar content being viewed by others
References
Bubenik GA, Bubenik AB, Schams D, Leatherland JF (1983) Circadian and circannual rhythms of LH, FSH, testosterone (T), prolactin, cortisol, T3 and T4 in plasma of mature, male white-tailed deer. Comp Biochem Physiol A 76:37–45
Bubenik GA, Schams D, White RG, Rowell J, Black J, Bartoš L (1998) Seasonal levels of metabolic hormones and substrates in male and female reindeer (Rangifer tarandus). Comp Biochem Physiol C 120:307–315
Christofoletti MD, Pereira RJG, Duarte JMB (2010) Influence of husbandry systems on physiological stress reactions of captive brown brocket (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus)—noninvasive analysis of fecal cortisol metabolites. Eur J Wildl Res, in press
Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16:809–814
Dehnhardt M, Clauss M, Lechner-Doll M, Meyer HH, Palme R (2001) Non-invasive monitoring of adrenocortical activity in roe deer (Capreolus capreolus) by measuring fecal cortisol metabolites. Gen Comp Endocrinol 123:111–120
Fahrig L (1997) Relative effects of habitat lossand fragmentation on population extinction. J Wildl Manag 61:603–610
Griffin JFT, Thomson AJ (1998) Farmed deer: a large animal model for stress. Domest Anim Endocrinol 15:445–456
Huber S, Palme R, Arnold W (2003a) Effects of season, sex, and sample collection on concentrations of fecal cortisol metabolites in red deer (Cervus elaphus). Gen Comp Endocrinol 130:48–54
Huber S, Palme R, Zenker W, Möstl E (2003b) Non-invasive monitoring of the adrenocortical response in red deer. J Wildl Manag 67:258–266
Huxel GR, Hastings A (1999) Habitat loss, fragmentation and restoration. Restor Ecol 7:309–315
Lexen E, El-Bahr SM, Sommerfeld-Stur I, Palme R, Möstl E (2008) Monitoring the adrenocortical response to disturbances in sheep by measuring glucocorticoid metabolites in the faeces. Wien Tierärztl Mschr–Vet Med Austria 95:64–71
Lindner HR (1972) Enterohepatic circulation and patterns of urinary excretion of cortisol metabolites in the ewe. J Endocrinol 52:19–20
Millspaugh JJ, Woods RJ, Hunt KE, Raedeke KJ, Brundige GC, Washburn BE, Wasser SK (2001) Fecal glucocorticoid assays and the physiological stress response in elk. Wildl Soc Bull 29:899–907
Millspaugh JJ, Washburn BE, Milanick MA, Beringer J, Hansen LP, Meyer TM (2002) Non-invasive techniques for stress assessment in white-tailed deer. Wildl Soc Bull 30:899–907
Mormède P, Andanson S, Auperin B, Beerda B, Guemene D, Malmkvist J, Manteca X, Manteuffel G, Prunet P, van Reenen CG, Richard S, Veissier I (2007) Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol Behav 92:317–339
Möstl E, Palme R (2002) Hormones as indicators of stress. Dom Anim Endocrinol 23:67–74
Müller DWH, Gaillard JM, Lackey LB, Hatt LM, Clauss M (2010) Comparing life expectancy of three deer species between captive and wild populations. Eur J Wildl Res, in press
Palme R (2005) Measuring fecal steroids: guidelines for practical application. Ann NY Acad Sci 1046:75–80
Palme R, Möstl E (1997) Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Z Saugetierkd–Int J Mammal Biol 62:192–197 (Suppl. 2)
Palme R, Fischer P, Schildorfer H, Ismail MN (1996) Excretion of infused 14C-steroid hormones via faeces and urine in domestic livestock. Anim Reprod Sci 43:43–63
Palme R, Robia C, Messmann S, Hofer J, Möstl E (1999) Measurement of faecal cortisol metabolites in ruminants: a non-invasive parameter of adrenocortical function. Wien Tierärztl Mschr 86:237–241
Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E (2005) Stress hormones in mammals and birds: comparative aspects regarding metabolism, excretion and noninvasive measurement in fecal samples. Trends in comparative endocrinology and neurobiology. Ann NY Acad Sci 1040:162–171
Pereira RJG, Duarte JMB, Negrão JA (2006) Effects of environmental conditions, human activity, reproduction, antler cycle and grouping on fecal glucocorticoids of free-ranging Pampas deer stags (Ozotoceros bezoarticus bezoarticus). Horm Behav 49:114–122
Pesenhofer G, Palme R, Pesenhofer RM, Kofler J (2006) Comparison of two methods of fixation during functional claw trimming–walk-in crush versus tilt table—in dairy cows using faecal cortisol metabolite concentrations and daily milk yield as parameters. Wien Tierärztl Mschr–Vet Med Austria 93:288–294
Rehnus M, Hackländer K, Palme R (2009) A non-invasive method for measuring glucocorticoid metabolites (GCM) in mountain hares (Lepus timidus). Eur J Wildl Res 55:615–620
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255
Taillon J, Côté SD (2008) Are faecal hormone levels linked to winter progression, diet quality and social rank in young ungulates? An experiment with white-tailed deer (Odocoileus virginianus) fawns. Behav Ecol Sociobiol 62:1591–1600
Thiel D, Jenni-Eiermann S, Braunisch V, Palme R, Jenni L (2008) Ski tourism affects habitat use and evokes a physiological stress response in capercaillie Tetrao urogallus: a new methodological approach. J Appl Ecol 54:845–853
Touma C, Palme R (2005) Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of a biological validation. Ann NY Acad Sci 1046:54–74
Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275
Acknowledgement
The study was supported by the grant of the Ministry of Science, Education and Sport of the Republic of Croatia, No. 053-0532400-2399, “Applied biomedical research of deer game”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Konjević, D., Janicki, Z., Slavica, A. et al. Non-invasive monitoring of adrenocortical activity in free-ranging fallow deer (Dama dama L.). Eur J Wildl Res 57, 77–81 (2011). https://doi.org/10.1007/s10344-010-0401-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-010-0401-1