Abstract
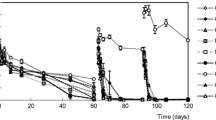
Whole-plant glasshouse experiments were conducted to examine herbicide resistance in selected populations of blackgrass (Alopecurus myosuroides HUDS.). Three populations with either non-target-site resistance (NTSR) or target-site resistance (TSR) showed reduced sensitivity to the herbicides fenoxaprop and mesosulfuron + iodosulfuron. Degradation and metabolism of these herbicides were investigated using a liquid chromatography followed by tandem mass spectrometry. Fenoxaprop degraded in sensitive and resistant populations within 144 h after treatment without significant differences among populations. Fenoxaprop-P, the acid metabolite of fenoxaprop, was found in all populations. The dynamics of fenoxaprop-P differed significantly with an enhanced degradation of the substance in NTSR populations. The metabolite 6-chlorobenzoxazol-2(3H)-one could be detected in all populations 2 h after treatment and degraded almost completely within 144 h.
The degradation of mesosulfuron + iodosulfuron lasted over 21 days. It was significantly faster in the NTSR than in the sensitive and the TSR populations. The metabolite metsulfuron was found 7 days after treatment in sensitive and resistant populations, without significant differences in the dynamics. The results clearly demonstrate that herbicide metabolism plays an important role in the evolution of herbicide resistant blackgrass populations.
Zusammenfassung
Ausgewählte Populationen von Ackerfuchsschwanz (Alopecurus myosuroides HUDS.) wurden hinsichtlich ihrer Herbizidresistenz im Gewächshaus untersucht. Drei Populationen, in denen die Resistenz entweder nicht-wirkortspezifisch (NTSR) oder wirkortspezifisch (TSR) begründet war, zeigten eine verminderte Sensitivität gegenüber den Herbiziden Fenoxaprop und Mesosulfuron + Iodosulfuron. Mittels Flüssigchromatographie mit Massenspektrometrie-Kopplung wurde der Abbau sowie der Metabolismus dieser Herbizide untersucht. Fenoxaprop wurde sowohl in der sensitiven als auch in den resistenten Populationen ohne signifikante Unterschiede innerhalb 144 h nach Applikation abgebaut. Fenoxaprop-P, die freie Säure von Fenoxaprop, wurde in allen Populationen, mit signifikanten Unterschieden in der Dynamik, nachgewiesen. Der Abbau von Fenoxaprop-P war in den resistenten, verstärkt in den NTSR Populationen, signifikant schneller. Zwei Stunden nach Applikation konnte der Metabolit 6-chlorobenzoxazol-2(3H)-one in allen Populationen nachgewiesen werden. Innerhalb von 144 h wurde dieser fast vollständig abgebaut. Der Abbau von Mesosulfuron + Iodosulfuron erfolgte innerhalb von 21 Tagen und war signifikant schneller in den NTSR Populationen als in der sensitiven und der TSR Population. Ohne signifikante Unterschiede in der Dynamik wurde der Metabolit Metsulfuron in allen Populationen 7 h nach Applikation nachgewiesen. Die Ergebnisse dieser Studie zeigen, dass der Metabolismus von Herbiziden eine wichtige Rolle in der Entwicklung von Herbizidresistenzen in A. myosuroides spielt.






Similar content being viewed by others
References
Anonymous (2014) Pesticide properties data base. http://sitem.herts.ac.uk/aeru/ppdb/en/. (last accessed 21 February 2015)
Bailly G, Dale R, Archer S, Wright D & Kaundun S (2012) Role of residual herbicides for the management of multiple herbicide resistance to ACCase and ALS inhibitors in a black-grass population. Crop Protect 34:96–103
Burton J, Gronwald J, Keith R, Somers D, Gengebach B & Wyse D (1991) Kinetics of inhibition of acetyl-coenzyme A carboxylase by sethoxydim and haloxyfop. Pest Biochem Physiol 39:100–109
Cocker KM, Moss SR & Coleman JO (1999) Multiple mechanisms of resistance to fenoxaprop-P-ethyl in United Kingdom and other European populations of herbicide-resistant Alopecurus myosuroides (black-grass). Pest Biochem Physiol 65:169–180
Cummins I, Bryant DN & Edwards R (2009) Safener responsiveness and multiple herbicide resistance in the weed black-grass (Alopecurus myosuroides). Plant Biotech J 7:807–820
Délye C, Gardin J, Boucansaud K, Chauvel B & Petit C (2011) Non-target-site-based resistance should be the centre of attention for herbicide resistance research: Alopecurus myosuroides as an illustration. Weed Res 51:433–437
Délye C, Jasieniuk M & LE Corre V (2013) Deciphering the evolution of herbicide resistance in weeds. Trends Gen 29:649–658
Development core team R (2010) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 17 Feb 2015
Gennari M, Vincenti M, Nègre M & Ambrosoli R (1995) Microbial metabolism of fenoxaprop-ethyl. Pest Sci 44:299–303
Gerhards R (2009) Unkrautbekämpfung in Winterweizen (Weed Control in Winter wheat). In: Christen O (ed) Winterweizen—Das Handbuch für Profis (Winter Wheat Handbook). DLG-Verlag, Frankfurt, pp 155–176
Heap I (2014a) Global perspective of herbicide-resistant weeds. Pest Manag Sci 70:1306–1315
Heap I (2014b) The international survey of herbicide resistant weeds. www.weedscience.org. Accessed 21 Feb 2015
Hoppe HH & Zacher H (1985) Inhibition of fatty acid biosynthesis in isolated bean and maize chloroplasts by herbicidal phenoxy-phenoxypropionic acid derivatives and structurally related compounds. Pest Biochem Physiol 24:298–305
Kaiser Y, Menegat A & Gerhards R (2013) Chlorophyll fluorescence imaging: a new method for rapid detection of herbicide resistance in Alopecurus myosuroides. Weed Res 53:399–406
King SR, Hagood JR ES, Bradley KW & Hatzios KK (2003) Absorption, translocation, and metabolism of AE F130060 03 in wheat, barley, and Italian ryegrass (Lolium multiflorum) with or without dicamba. Weed Sci 51:509–514
Knezevic SZSZ (2007) Utilizing R software package for dose-response studies: the concept and data analysis. Weed Tech 21:840–848
Kobek K, Focke M & Lichtenthaler HK (1988) Fatty-acid biosynthesis and acetyl-CoA carboxylase as a target of diclofop, fenoxaprop and other aryloxy-phenoxy-propionic acid herbicides. Z Naturforsch C 43:47–54
Kobek K & Lichtenthaler HK (1989) Inhibition of de novo fatty acid biosynthesis in isolated etioplasts by herbicides. Z Naturforsch C 44:669–672
Kocher H (2005) Mesosulfuron-methyl and combination partner iodosulfuron-methyl-sodium-mode of herbicidal action. PflSchutz Nachr-Bayer-Engl ed 58:179–194
Krysiak M, Gawronski S, Kierzek R & Adamczewski K (2011) Molecular basis of blackgrass (Alopecurus myosuroides Huds.) resistance to sulfonylurea herbicides. J Plant Prot Res 51:130–133
Moss SR, Perryman SA & Tatnell LV (2009) Managing herbicide-resistant blackgrass (Alopecurus myosuroides): theory and practice. Weed Tech 21:300–309
Pinheiro JC & Bates DM (2000) Appendix C5– SSfol—First order compartment model. In: Pinheiro JC, Bates DM (eds) Mixed-effects Models in S and S-PLUS, 3rd edn. Springer, New York, pp 516–517
Powles SB & Yu Q (2010) Evolution in action: plants resistant to herbicides. Ann rev plant biol 61:317–347
Ritz C & Streibig JC (2005) Bioassay analysis using R. J Stat Software 12:1–22
Rubin B (1997) Herbicide resistance outside North America and Europe: causes and significance. In: De Prado R, Jorrin J, Garcia-Torres L (eds) Weed and Crop Resistance to Herbicides. Springer, Dordrecht
Singh SB, Das TK & Kulshrestha G (2013) Persistence of herbicide fenoxaprop ethyl and its acid metabolite in soil and wheat crop under Indian tropical conditions. J Environ Sci Health, Part B 48:324–330
Tal A, Romano M, Stephenson G, Schwan A & Hall J (1993) Glutathione conjugation: a detoxification pathway for fenoxaprop-ethyl in barley, crabgrass, oat, and wheat. Pest Biochem Physiol 46:190–199
Tomlin CD (ed) (2003) The pesticide manual, 13th edn. British Crop Prot Council, Hampshire
Yaacoby T, Hall JC & Stephenson G (1991) Influence of fenchlorazole-ethyl on the metabolism of fenoxaprop-ethyl in wheat, barley, and crabgrass. Pest Biochem Physiol 41:296–304
Acknowledgments
This project was funded by the DFG (Deutsche Forschungsgemeinschaft). The authors want to thank Dr. Frank Walker for the supervision and the guidance concerning the chemical preparation of plant samples and the LC/MS-MS measurements. We would like to thank Alexandra Heyn, Birgit Höglinger and the Central Chemical-Analytical Laboratory in Hohenheim for the assistance in the laboratory. Many thanks to Dr. Martin Hess and Dr. Jan Petersen for kindly providing seed samples for this study. Thanks to Bayer Crop Science for providing the analytical standard of fenoxaprop and mesosulfuron + iodosulfuron.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nomenclature fenoxaprop; mesosulfuron + iodosulfuron; blackgrass, Alopecurus myosuroides Huds., ALOMY
Rights and permissions
About this article
Cite this article
Kaiser, Y., Gerhards, R. Degradation and Metabolism of Fenoxaprop and Mesosulfuron + Iodosulfuron in Multiple Resistant Blackgrass (Alopecurus myosuroides) . Gesunde Pflanzen 67, 109–117 (2015). https://doi.org/10.1007/s10343-015-0343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-015-0343-3