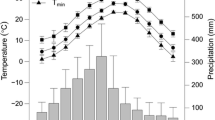
Abstract
The climatic conditions predicted for the twenty-first century may aggravate the extent and impacts of plant invasions, by favouring those invaders more adapted to altered conditions or by hampering the native flora. We aim to predict the fate of native and invasive tree species in the oak forests of Northwest Spain, where the exotic invaders Acacia dealbata and Eucalyptus globulus co-occur with the natives Quercus robur and Quercus pyrenaica and the naturalized Pinus pinaster. We selected adult, dominant trees of each species, collected increment cores, measured the ring width and estimated the basal area increment (BAI, cm2 year−1). Climate/growth models were built by using linear mixed-effect models, where the previous-year BAI and seasonal temperature and precipitation were the fixed factors and the individual the random factor. These models were run to project the fate of studied species in the A2 and B2 CO2 emission scenarios until 2100. The models explained over 50 % of BAI variance in all species but E. globulus, where growth probably occurs whenever a minimum environmental requirement is met. Warm autumns favoured BAI of both natives, probably due to an extension of leaf lifespan, but hampered A. dealbata and P. pinaster BAI, maybe because of water imbalance and/or the depletion of carbon reserves. The projections yielded a positive BAI trend for both Quercus along the twenty-first century, but negative for the invader A. dealbata and clearly declining for the naturalized P. pinaster. Our results disagree with previous literature pointing at climate change as a driver of invasive species’ success and call for further studies regarding the effect of climate change on co-occurring natives and invaders.



Similar content being viewed by others
References
Alía R, Moro J, Denis JB (1997) Performance of Pinus pinaster provenances in Spain: interpretation of the genotype by environment interaction. Can J For Res 27:1548–1559. doi:10.1139/cjfr-27-10-1548
Baquedano FJ, Castillo J (2007) Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis, and Juniperus phoenicea. Photosynthetica 45:229–238. doi:10.1007/s11099-007-0037-x
Barbaroux C, Bréda N (2002) Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 221:201–1210. doi:10.1093/treephys/22.17.1201
Barlow BA (1981) The Australian flora: its origin and evolution. In: George SA (ed) Flora of Australia. Vol. 1: introduction. Australian Government Publishing Service, Canberra, pp 25–75
Béllard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecol Lett 15:365–377. doi:10.1111/j.1461-0248.2011.01736.x
Blanco Castro E, Casado González MA, Costa Tenorio M, Escribano Bombín R, García Antón M, Génova Fuster M, Gómez Manzaneque MA, Gómez Manzaneque F, Moreno Saiz JC, Morla Juaristi C, Regato Pajares P, Sainz Ollero H (2005) Los bosques ibéricos: una interpretación geobotánica. Planeta, Barcelona
Borralho N, Araújo MC, Pereira JS (1989) Influence of water supply on crown structure and production of three clones of Eucalyptus globulus in the juvenile phase. In: Kreeb KH, Ritcher H, Hincley TM (eds) Structural and functional responses to environmental stresses: water shortage. XIV International Botanical Congress, Berlin, 24 July-1 August 1987, pp 181–190
Bowman D, Prior LD (2005) Why do evergreen trees dominate the Australian seasonal tropics? Aust J Bot 53:379–399. doi:10.1071/BT05022
Bréda N, Granier A (1996) Intra- and interannual variations of transpiration, leaf area index and radial growth of a sessile oak stand (Quercus petraea). Ann For Sci 53:521–536. doi:10.1051/forest:19960232
Brunet M, Casado MJ, De Castro M, Galán P, López JA, Martín JM, Pastor A, Petisco E, Ramos P, Ribalaygua J, Rodríguez E, Sanz I, Torres L (2009) Generación de escenarios regionalizados de cambio climático para España. AEMET, Madrid
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, Heidelberg
Candel-Pérez D, Linares JC, Viñegla B, Lucas-Borja ME (2012) Assessing climate–growth relationships under contrasting stands of co-occurring Iberian pines along an altitudinal gradient. For Ecol Manag 274:48–57. doi:10.1016/j.foreco.2012.02.010
Canellas I, Del Rio M, Roig S, Montero G (2004) Growth response to thinning in Quercus pyrenaica Willd. coppice stands in Spanish central mountain. Ann For Sci 61(3):243–250. doi:10.1051/forest:2004017
Castro-Díez P, Godoy O, Saldaña A, Richardson DM (2011) Predicting invasiveness of Australian acacias on the basis of their native climatic affinities, life history traits and human use. Divers Distrib 17(5):934–945. doi:10.1111/j.1472-4642.2011.00778.x
Ceballos L, Ruiz de la Torre J (1979) Árboles y arbustos. ETSIM, Madrid
Chambel MR, Climent J, Alía R (2007) Divergence among species and populations of Mediterranean pines in biomass allocation of seedlings grown under two watering regimes. Ann For Sci 64:87–97. doi:10.1051/forest:2006092
Chen PY, Welsh C, Hamann A (2010) Geographic variation in growth response of Douglas-fir to interannual climate variability and projected climate change. Glob Change Biol 16:3374–3385. doi:10.1111/j.1365-2486.2010.02166.x
Chhin S, Wang GG (2005) The effect of sampling height on dendroclimatic analysis. Dendrochronologia 23:47–55. doi:10.1016/j.dendro.2005.07.003
Closset-Kopp D, Saguez R, Decocq G (2012) Differential growth patterns and fitness may explain contrasted performances of the invasive Prunus serotina in its exotic range. Biol Invasions 13(6):1341–1355. doi:10.1007/s10530-010-9893-6
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptative? A meta-analysis. Ecol Lett 14:419–431
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invisibility. J Ecol 88:528–534
Dietz H, Ullmann I (1998) Ecological application of ‘Herbchronology’: comparative stand age structure analyses of the invasive plant Bunias orientalis L. Ann Bot 82(4):471–480. doi:10.1111/j.1461-0248.2011.01596.x
Diez JM, D´Antonio CM, Dukes JS, Grosholz ED, Olden JD, Sorte CJB (2012) Will extreme climatic events facilitate biological invasions? Front Ecol Environ 10:249–257. doi:10.1890/110137
Dougherty PM, Teskey RO, Phelps JE, Hinckley TM (1979) Net photosynthesis and early growth trends of a dominant White Oak (Quercus alba L.). Plant Physiol 64:930–935. doi:10.1104/pp.64.6.930
Drobyshev I, Linderson H, Sonesson K (2007) Relationship between crown condition and tree diameter growth in southern Swedish oaks. Environ Monit Assess 128:61–73. doi:10.1007/s10661-006-9415-2
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14(4):135–139. doi:10.1016/S0169-5347(98)01554-7
Felicísimo AM (2011) Impactos, vulnerabilidad y adaptación al cambio climático de la biodiversidad española. 1. Flora y vegetación. Oficina Española de Cambio Climático, Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid
Felicísimo AM, Muñoz J, Mateo RG (2012) Vulnerabilidad de la flora y vegetación españolas ante el cambio climático y propuestas de actuación. Ecosistemas 21(3):1–6
Feliksik E, Wilczyński S (2009) The effect of climate on tree-ring chronologies of native and nonnative tree species growing under homogenous site conditions. Geochronometria 33(1):49–57. doi:10.2478/v10003-009-0006-4
Fotelli MN, Radoglou KM, Constantinidou HIA (2000) Water stress responses of seedlings of four Mediterranean oak species. Tree Physiol 20(16):1065–1075
Fritts HC (1976) Tree rings and climate. Academic Press, London
Fuentes-Ramírez A, Pauchard A, Cavieres LA, García RA (2011) Survival and growth of Acacia dealbata vs. native trees across an invasion front in southcentral Chile. For Ecol Manag 261:1003–1009. doi:10.1016/j.foreco.2010.12.018
Gieger T, Thomas FM (2002) Effects of defoliation and drought stress on biomass partitioning and water relations of Quercus robur and Quercus petraea. Basic Appl Ecol 3(2):171–181. doi:10.1078/1439-1791-00091
Gómez-Miguel V (2006) Geología, Geomorfología y Edafología, Monografías del Atlas Nacional de España. Instituto Geográfico Nacional, Madrid
González-Muñoz N, Castro-Díez P, Fierro-Brunnenmeister N (2011) Establishment success of coexisting native and exotic trees under an experimental gradient of irradiance and soil moisture. Environ Manag 48:764–773. doi:10.1007/s00267-011-9731-3
González-Muñoz N, Costa-Tenorio M, Espigares T (2012) Invasion of alien Acacia dealbata on Spanish Quercus robur forests: impacts on soils and vegetation. For Ecol Manag 269:214–221. doi:10.1016/j.foreco.2011.12.026
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res 57(2):205–221. doi:10.1016/j.dendro.2010.12.002
Grotkopp E, Rejmánek M, Rost TL (2002) Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159:396–419. doi:10.1086/338995
Grotkopp E, Erskine-Ogden J, Rejmánek M (2010) Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits. J Appl Ecol 47(6):1320–1328. doi:10.1111/j.1365-2664.2010.01878.x
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8(9):993–1009. doi:10.1111/j.1461-0248.2005.00792.x
Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS (2008) Five potential consequences of climate change for invasive species. Conserv Biol 22(3):534–543. doi:10.1111/j.1523-1739.2008.00951.x
Hernández-Santana V, Martínez-Fernández J, Morán JC, Cano A (2008) Response of Quercus pyrenaica (melojo oak) to soil water deficit: a case study in Spain. Eur J For Res 127:369–378. doi:10.1007/s10342-008-0214-x
Hinckley TM, Lassoie JP (1981) Radial growth in conifers and deciduous trees: a comparison. Mitteilungen der forstlichen Bundesversuchsanstalt Wien. 142:17–56
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Huang D, Haack RA, Zhang R (2011) Does global warming increase establishment rates of invasive alien species? A centurial time series analysis. PLoS One 6(9):e24733. doi:10.1371/journal.pone.0024733
IPCC (2007) Climate change 2007, the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IPCC (2013) Climate change 2013, the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jacobs MR (1955) Growth habits of the eucalypts. Commonwealth Government Printer, Canberra
Jato V, Rodríguez-Rajo FJ, Méndez J, Aira MJ (2002) Phenological behaviour of Quercus in Ourense (NW Spain) and its relationship with the atmospheric pollen season. Int J Biometeorol 46:176–184. doi:10.1007/s00484-002-0132-4
Joslin JD, Wolfe MH, Hanson PJ (2000) Effects of altered water regimes on forest root systems. New Phytol 147(1):117–129. doi:10.1046/j.1469-8137.2000.00692.x
Kuster TM, Arend M, Günthardt-Goerg MS, Schulin R (2013) Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant Soil 369:61–71. doi:10.1007/s11104-012-1541-8
Lamarque LJ, Delzon S, Lortie CJ (2011) Tree invasions: a comparative test of the dominant hypotheses and functional traits. Biol Invasions 13:1969–1989. doi:10.1007/s10530-011-0015-x
Le Maitre DC, Versfeld DB, Chapman RA (2000) The impact of invading alien plants on surface water resources in South Africa: a preliminary assessment. Water Res Comm 26(3):397–408
Lebourgeois F (2000) Climatic signals in earlywood, latewood and total ring width of Corsican pine from western France. Ann For Sci 57(2):155–164. doi:10.1051/forest:2000166
Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771. doi:10.1126/science.1156831
Levitt J (1980) Responses of plants to environmental stresses. Academic Press, New York
Li JB, Xu CY, Griffin KL, Schuster WSF (2008) Dendrochronological potential of Japanese barberry (Berberis thunbergii): a case study in the Black Rock Forest, New York. Tree-Ring Res 64(2):115–124. doi:10.3959/2008-5.1
Linares JC, Covelo F, Carreira JA, Merino J (2012) Phenological and water-use patterns underlying maximum growing season length at the highest elevations: implications under climate change. Tree Physiol 32:161–170. doi:10.1093/treephys/tps003
Lodewick JE (1928) Seasonal activity of the cambium in some northeastern trees. Bull NY State Coll For 1(2):1–87
Lorenzo P, Pazos-Malvido E, González L, Reigosa M (2008) Allelopathic interference of invasive Acacia dealbata: physiological effects. Allelopath J 22:452–462. doi:10.1007/s11258-010-9831-9
Lorenzo P, González L, Reigosa MJ (2010) The genus Acacia as invader: the characteristic case of Acacia dealbata link in Europe. Ann For Sci 67:101. doi:10.1051/forest/2009082
Lupi C, Rossi S, Vieira J, Morin H, Deslauriers A (2013) Assessment of xylem phenology: a first attempt to verify its accuracy and precision. Tree Physiol 34(1):87–93. doi:10.1093/treephys/tpt108
Macova M (2008) Dendroclimatological comparison of native Pinus sylvestris and invasive Pinus strobus in different habitats in the Czech Republic. Preslia 80(3):277–289
May BM, Attiwill PM (2003) Nitrogen-fixation by Acacia dealbata and changes in soil properties 5 years after mechanical disturbance or slash-burning following timber harvest. For Ecol Manag 181:339–355. doi:10.1016/S0378-1127(03)00006-9
McKee TB, Doesken NJ, Kleist J (1993) The relationship of drought frequency and duration to time scales. In: 8th conference on applied climatology 17–22 January, Anaheim, CA, pp 179–184
Mediavilla S, Escudero A (2003) Stomatal responses to drought at a Mediterranean site: a comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol 23:987–996
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659. doi:10.1038/17709
Mooney HA, Hoobs RJ (2000) Global change and invasive species. Where do we go from here? In: Mooney HA, Hoobs RJ (eds) Invasive species in a changing world. Island Press, Washington, pp 425–435
Morgan EC (2012) Stand dynamics of a 46-year invasion by Phellodendron amurense rupr. in an Eastern North American Forest. Castanea 77(1):21–27. doi:10.2179/11-039
Mortenson SG, Mack RN (2006) The fate of alien conifers in long-term plantings in the USA. Divers Distrib 12(4):456–466. doi:10.1111/j.1366-9516.2006.00274.x
Nakicenovic N, Alcamo J, Davis G, Vries BD, Fenhann J, Gaffin S, Gregory K, Grübler A, Yong Jung T, Kram T, La Rovere EL, Michaelis L, Mori S, Morita T, Pepper W, Pitcher H, Price L, Riahi K, Roehrl A, Rogner HH, Sankovski A, Schlesinger M, Shukla P, Smith S, Swart R, van Rooijen S, Victor N, Dadi Z (2000) Special report on emissions scenarios: a special report of working group III of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
O’Neill GA, Hamann A, Wang T (2008) Accounting for population variation improves estimates of the impact of climate change on species’ growth and distribution. J Appl Ecol 45:1040–1049. doi:10.1111/j.1365-2664.2008.01472.x
Ogden J (1982) Australasia. In: Hughes MK, Kelly PM, Pilcher JR, LaMarche VCJ (eds) Climate from tree rings. Cambridge University Press, Cambridge, pp 90–104
Osório J, Osório ML, Chaves MM, Pereira JS (1998) Water deficits are more important in delaying growth than in changing patterns of carbon allocation in Eucalyptus globulus. Tree Physiol 18(6):363–373. doi:10.1093/treephys/18.6.363
Pallardy SG, Kozlowsky TT (2008) Physiology of woody plants. Elsevier, Burlington
Parmesan C (2006) Ecological and evolutionary responses to recent climate Change. Annu Rev Ecol Evol Syst 37:637–669. doi:10.1146/annurev.ecolsys.37.091305.110100
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. doi:10.1038/nature01286
Paton DM (1978) Eucalyptus physiology. I. Photoperiodic responses. Aust J Bot 26:633–642. doi:10.1071/BT9780633
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeogr 12:361–371. doi:10.1046/j.1466-822X.2003.00042.x
Peñuelas J, Boada M (2003) A global change-induced biome shift in the Montseny Mountains (NE Spain). Glob Change Biol 9:131–140. doi:10.1046/j.1365-2486.2003.00566.x
Peñuelas J, Filella I (2001) Phenology: responses to a warming world. Science 294:793–795. doi:10.1126/science.1066860
Peterson DW, Peterson DL, Ettl GJ (2002) Growth responses of subalpine fir (Abies lasiocarpa) to climatic variability in the Pacific Northwest. Can J For Res 32:1503–1517. doi:10.1139/X02-072
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http:www.r-project.org
Rejmánek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661. doi:10.2307/2265768
Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107. doi:10.1046/j.1472-4642.2000.00083.x
Rinn F (1996) TSAP (Time Series Analysis and Presentation) Version 3.0. Heidelberg, Germany
Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA (2003) Fingerprints of global warming on wild animals and plants. Nature 421:57–60. doi:10.1038/nature01309
Rozas V, Lamas S, García-González I (2009) Differential tree-growth responses to local and large-scale climatic variation in two Pinus and two Quercus species in Northwest Spain. Ecoscience 16(3):299–310. doi:10.2980/16-3-3212
Rubino DL, McCarthy BC (2000) Dendroclimatological analysis of White Oak (Quercus alba L., Fagaceae) from an old-growth forest of southeastern Ohio, USA. J Torrey Bot Soc 127:240–250. doi:10.2307/3088761
Ruiz-Labourdette D, Nogues-Bravo D, Ollero HS, Schmitz MF, Pineda FD (2012) Forest composition in Mediterranean mountains is projected to shift along the entire elevational gradient under climate change. J Biogeogr 39(1):162–176. doi:10.1111/j.1365-2699.2011.02592.x
Ryan MG, Binkley D, Fownes JH (1997) Age-related decline in forest productivity: pattern and process. Adv Ecol Res 27:213–262
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332. doi:10.1146/annurev.ecolsys.32.081501.114037
Sanz Elorza M, Dana Sánchez ED, Sobrino Vespertinas E (2004) Atlas de plantas alóctonas invasoras en España. Ministerio de Medio Ambiente, Madrid
Scurfield G (1961) The effects of temperature and day length on species of Eucalyptus. Aust J Bot 9:37–56. doi:10.1071/BT9610037
Silva JS, Rego FC, Martins-Louçao MA (2003) Root distribution of Mediterranean woody plants. Introducing a new empirical model. Plant Biosyst 137(1):63–72. doi:10.1080/11263500312331351341
Sisó S, Camarero JJ, Gil-Pelegrín E (2001) Relationship between hydraulic resistance and leaf morphology in broadleaf Quercus species: a new interpretation of leaf lobation. For Ecol Manag 15:341–345. doi:10.1007/s004680100110
Soil Survey Staff (2010) Keys to soil taxonomy. USDA-Natural Resources Conservation Service, Washington
Thomas FM, Gausling T (2000) Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann For Sci 57(4):325–333. doi:10.1051/forest:2000123
Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, Hickler T, Midgley GF, Peterson J, Churr FM, Sykes MT, Zimmermann NE (2008) Predicting global change impacts on plant species’ distributions: future challenges. Perspect Plant Ecol Evol Syst 9(3–4):137–152. doi:10.1016/j.ppees.2007.09.004
Timbal J, Aussenac G (1996) An overview of ecology and silviculture of indigenous oaks in France. Ann Sci For 53(2–3):649–661. doi:10.1051/forest:19960243
Turnbull JW (1999) Eucalypt plantations. New For 17(1–3):37–52
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. doi:10.1111/j.1461-0248.2009.01418.x
Vanhellemont M, Baeten L, Verbeeck H, Hermy M, Verheyen K (2011) Long-term scenarios of the invasive black cherry in pine-oak forest: impact of regeneration success. Acta Oecol 37:203–211. doi:10.1016/j.actao.2011.02.003
Vieira J, Campelo F, Nabais C (2009) Age-dependent responses of tree-ring growth and intra-annual density fluctuations of Pinus pinaster to Mediterranean climate. Trees 23:257–265. doi:10.1007/s00468-008-0273-0
Vilà M, Weiner J (2004) Are invasive plant species better competitors tan native plant species? Evidence from pair-wise experiments. Oikos 105:229–238. doi:10.1111/j.0030-1299.2004.12682.x
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416(28):389–395. doi:10.1038/416389a
Walther GR, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarosík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24(12):686–693. doi:10.1016/j.tree.2009.06.008
Wareing PF (1951) Growth studies in woody species IV. The initiation of cambial activity in ring-porous trees. Physiol Plant 4:546–562
Weber E (2003) Invasive plant species of the world. A reference guide to environmental weeds. CABI Publishing, Zurich
Woodward F (1987) Climate and plant distribution. Cambridge University Press, Cambridge
Worbes M (2002) One hundred years of tree ring research in the tropics -a brief history and an outlook to future challenges. Dendrochronologia 20:217–231
Yamaguchi DK (1991) A simple method for cross-dating increment cores from living trees. Can J For Res 21:414–416. doi:10.1139/x91-053
Yeh HY, Wensel LC (2000) The relationship between tree diameter growth and climate for coniferous species in northern California. Can J For Res 30:1463–1471. doi:10.1139/x00-074
Zalba SM, Cuevas Y, Boó RM (2008) Invasion of Pinus halepensis Mill. following a wildfire in an Argentine grassland nature reserve. J Environ Manag 88:539–546. doi:10.1016/j.jenvman.2007.03.018
Zas R, Merlo E, Fernández-López J (2004) Genotype x environment interaction in Maritime pine families in Galicia, Northwest Spain. Silvae Genet 53:175–182. doi:10.1051/forest/2010025
Acknowledgments
This study was supported by the Projects CGL2010-16388/BOS of the Spanish Ministry of Science and Innovation, POII10-0179-4700 of Junta de Comunidades de Castilla-La Mancha and by the REMEDINAL-2 network S2009/AMB-1783 (Comunidad de Madrid). Noelia González-Muñoz was supported by a postdoctoral contract of the REMEDINAL-2 network and of Universidad de Alcalá. Juan Carlos Linares thanks the support provided by a research grant of the University Pablo de Olavide, APP2D09497. We got inspired by discussions during meetings of the COST Action FP1106, STReESS. We thank Margarita Costa-Tenorio and Evelyn Beliën for her valuable help in the field work and Ellen Wilderink for her help at the laboratory measurements. We acknowledge the Forest Ecology and Forest Management Group at Wageningen University for allowing us using their facilities and for the fruitful discussions during Noelia González-Muñoz short stays. These stays were funded by The C. T. de Wit Graduate School for Production Ecology and Resource Conservation of Wageningen University and by a FPI-Fellowship of the Government of Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dr. Christian Ammer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10342_2014_823_MOESM1_ESM.docx
Appendix A. Detail of the last rings formed by the invaders Acacia dealbata (A) and Eucalyptus globulus (B), the naturalized Pinus pinaster (C) and the natives Quercus pyrenaica (D) and Quercus robur (E). Samples collected in July 2008 (DOCX 316 kb)
10342_2014_823_MOESM2_ESM.docx
Appendix B. SPI and mean annual temperature (ºC) from 1990 to 2100 for the study area (Orense, Spain) and for the emission scenarios A2 and B2 (IPCC 2007) according to the General Circulation Models CGCM2 and ECHAM4 and projected by the Instituto Nacional de Meteorología (AEMET, Government of Spain). The x-axis of the annual mean temperature is adjusted on the average temperature for the period 1961–1990 (DOCX 1889 kb)
10342_2014_823_MOESM3_ESM.docx
Appendix C. Maximum and minimum values of tree age (number of ring measured) and diameter at breast height (DBH, cm) found in the sampled trees of each species. Maximum, minimum and average ± ES BAI (cm2 year−1) of each species along the model-calibration period (DOCX 15 kb)
10342_2014_823_MOESM5_ESM.docx
Appendix E. Statistical results of fitting a logistic function to the tree age (estimated as number of rings measured) to the sampled trees of Acacia dealbata and Quercus robur. Asym: asymptote; xmid: x value at the inflection point of the curve; scal: numeric scale parameter on the data axis. Statistical results of fitting a polynomial function to the age of the sampled trees of Pinus pinaster (second degree polynomial) and Quercus pyrenaica (fourth degree polynomial) (DOCX 15 kb)
10342_2014_823_MOESM6_ESM.docx
Appendix F. Model selection criteria for the first-order autocorrelation structure of the BAI (BAIp) in each species. The selected model is highlighted in bold characters. K, number of explaining variables plus one constant plus the error; AICc, Akaike information criterion corrected for small samples; ΔAICc, difference in AICc with respect to the best model; Wi, relative probability to be the best model for the observed data (DOCX 15 kb)
10342_2014_823_MOESM7_ESM.docx
Appendix G. Summary graphs of the fitted linear mixed models in each species for BAI (Basal Area Increment, cm2 year−1). First row: predicted vs. observed values of BAI. Second row: residuals vs. predicted values by the linear mixed models. Third to fifth row: residuals values vs. raw values of each independent variable. BAIp, first-order autocorrelation structure of BAI; tree age at sampling height (number of rings contained and measured in each corer); time (Calendar Year) (DOCX 398 kb)
Rights and permissions
About this article
Cite this article
González-Muñoz, N., Linares, J.C., Castro-Díez, P. et al. Predicting climate change impacts on native and invasive tree species using radial growth and twenty-first century climate scenarios. Eur J Forest Res 133, 1073–1086 (2014). https://doi.org/10.1007/s10342-014-0823-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-014-0823-5