Abstract
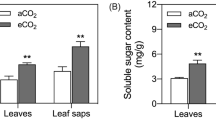
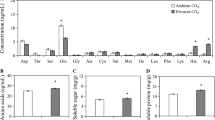
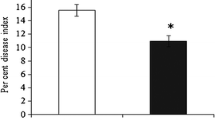
Elevated CO2 can alter plant resistance against insect herbivores. In this study, we investigated the effect of elevated CO2 on the callose synthesis involved in the resistance of Phaseolus vulgaris against Frankliniella occidentalis, which is one of the most important invasive insect pests worldwide. Elevated CO2 elevated the silver damage, callose deposition, and the expression level of CalS3 and CalS10 genes involved in callose synthase (CalS) in thrips-infested bean leaves, while reducing PR2 gene expression related to the hydrolysis of callose. In addition, both infestation by thrips and mechanical damage increased the callose deposition in leaves and induced CalS and β-1,3-glucanases (BG) expression at both transcriptional and translational levels. Under ambient CO2, callose content in the mechanically damaged plants (MDPs) and thrips-infested plants (TIPs) was positively correlated with CalS activity and the expression level of CalS3 and CalS10; BG activity was positively correlated with PR2 gene expression. Under elevated CO2, callose content in MDPs and TIPs was negatively correlated with BG activity which also negatively correlated with the expression level of CalS10 and PR2. F. occidentalis feeding can induce callose synthesis and deposition in P. vulgaris leaves, especially under elevated CO2. Specifically, genes associated with CalS defense are up-regulated, while the expression level of PR2 gene is downregulated. These results suggest that elevated CO2 can modulate callose synthesis leading to a higher level of resistance in host plants against thrips infestation.







Similar content being viewed by others
References
Abe H, Ohnishi J, Narusaka M, Seo S et al (2008) Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol 49:68–80
Abramoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Aidemark M, Andersson CJ, Rasmusson AG, Widell S (2009) Regulation of callose synthase activity in situ in alamethicin-permeabilized Arabidopsis and tobacco suspension cells. BMC Plant Biol 9:27
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant, Cell Environ 30:258–270
Alborn HT, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, Tumlinson JH (1997) An elicitor of plant volatiles from beet armyworm oral secretion. Science 276:945–949
Arimura G, Kost C, Boland W (2005) Herbivore-induced, indirect plant defences. BBA-Mol Cell Biol L 1734:91–111
Arimura G, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Bloom AJ, Burger M, Asensio JSR, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903
Chen FJ, Ge F, Liu XH (2004) Responses of cotton to elevated CO2 and the effects on cotton aphid occurrences. Acta Ecol Sin 24:991–996
Cong C, Zhi J, Xie L, Gao H (2013) Effects of Franklinella occidentalis feeding on the chlorophyll and nutrients in the leaves of Phaselous vulgaris. Plant Prot 39:20–24
Consales F, Schweizer F, Erb M, Gouhier-Darimont C et al (2012) Insect oral secretions suppress wound-induced responses in Arabidopsis. J Exp Bot 63:727–737
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Dong X, Hong Z, Chatterjee J, Kim S, Verma DP (2008) Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229:87–98
Donofrio NM, Delaney TP (2001) Abnormal callose response phenotype and hypersusceptibility to Peronospora parasitica in defense-compromised Arabidopsis nim1-1 and salicylate hydroxylase-expressing plants. Mol Plant Microbe Interact 14:439–450
Ellinger D, Naumann M, Falter C, Zwikowics C, Voigt CA (2013) Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol 161:1433–1444
Escobar-Bravo Klinkhamer PGL, Leiss KA (2017) Induction of Jasmonic Acid-associated defenses by thrips alters host suitability for conspecifics and correlates with increased trichome densities in tomato. Plant Cell Physiol 58:622–634
Eshraghi L, Anderson JP, Aryamanesh N, McComb JA, Shearer B, Hardy GEJ (2014) Defence signalling pathways involved in plant resistance and phosphite-mediated control of Phytophthora cinnamomic. Plant Mol Biol Rep 32:342–356
Farrokhi N, Burton RA, Brownfield L, Hrmova M et al (2006) Plant cell wall biosynthesis: genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol J 4:145–167
Fromme P, Melkozernov A, Jordan P, Krauss N (2003) Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. FEBS Lett 555:40–44
Galis I, Gaquerel E, Pandey SP, Baldwin IT (2009) Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ 32:617–627
Gindro K, Pezet R, Viret O (2003) Histological study of the responses of two Vitis vinifera cultivars (resistant and susceptible) to Plasmopara vitivola infections. Plant Physiol Biochem 41:846–853
Guerenstein PG, Hildebrand JG (2008) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53:161–178
Guo H, Sun Y, Li Y, Tong B et al (2013) Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Global Change Biol 19:3210–3223
Guseman JM, Lee JS, Bogenschutz NL, Peterson KM et al (2010) Dysregulation of cell-to-cell connectivity and stomatal patterning by loss-of-function mutation in Arabidopsis chorus (glucan synthase-like 8). Development 137:1731–1741
Hao P, Liu C, Wang Y, Chen R et al (2008) Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol 146:1810–1820
Inbar M, Doostdar H, Leibee GL, Mayer RT (1999) The role of plant rapidly induced responses in asymmetric interspecific interactions among insect herbivores. J Chem Ecol 25:1961–1979
IPCC (2014) Climate change 2014: synthesis report. In: Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva
Jackson RB, Cook CW, Pippen JS, Palmer SM (2009) Increased belowground biomass and soil CO2 fluxes after a decade of carbon dioxide enrichment in a warm-temperate forest. Ecology 90:3352–3366
Jimoh MA, Kaehler S, Botha CEJ (2013) Increased feeding damage under elevated CO2: the case of the Russian wheat aphid. S Afr J Bot 84:30–37
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347
Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144
Kirk WDJ, Terry LI (2003) The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agric Forest Entomol 5:301–310
Leiss F, Koper E, Hein I, Fouquet W et al (2009) Characterization of dendritic spines in the Drosophila central nervous system. Dev Neurobiol 69:221–234
Li J, Fang L, Lv Z, Zhang Z (2008) Relationship between the resistance against aphids and soluble sugar content in cotton. Plant Protection 34:26–30
Liu JY, Qian L, Jiang XC, He SQ, Li ZY, Gui FR (2014) Effects of elevated CO2 concentration on the activities of detoxifying enzymes and protective enzymes in adults of Frankliniella occidentalis and Frankliniella intonsa (Thysanoptera: Thripidae). Acta Entomol Sin 57:754–761
Massad TJ, Dyer LA (2010) A meta-analysis of the effects of global environmental change on plant–herbivore interactions. Arthropod-Plant Interact 4:181–188
Mayer RT, Inbar M, McKenzie CL, Shatters R et al (2002) Multitrophic interactions of the silverleaf whitefly, host plants, competing herbivores, and phytopathogens. Arch Insect Biochem 51:151–169
Morse JG, Hoddle MS (2006) Invasion biology of thrips. Annu Rev Entomol 51:67–89
Nishikawa Y, Quittnat F, Stedman TT, Voelker DR et al (2005) Host cell lipids control cholesteryl ester synthesis and storage in intracellular Toxoplasma. Cell Microbiol 7:849–867
Oide S, Bejai S, Staal J, Guan N, Kaliff M, Dixelius C (2013) A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol 200:1187–1199
Park DS, Lee SK, Lee JH, Song MY et al (2007) The identification of candidate rice genes that confer resistance to the brown planthopper (Nilaparvata lugens) through representational difference analysis. Theor Appl Genet 115:537–547
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qian L, Chen FJ, Liu JN, He SQ et al (2017) Effects of elevated CO2 on life-history traits of three successive generations of Frankliniella occidentalis and F. intonsa on kidney bean, Phaseolus vulgaris. Entomol Exp Appl. https://doi.org/10.1111/eea.12606
Qian L, He S, Liu X, Huang Z, Chen F, Gui F (2018) Effect of elevated CO2 on the interaction between invasive thrips, Frankliniella occidentalis, and its host kidney bean, Phaseolus vulgaris. Pest Manag Sci. https://doi.org/10.1002/ps.5064
Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12:707–720
Richmond TA, Somerville CR (2000) The cellulose synthase superfamily. Plant Physiol 124:495–498
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Schmutz J, Cannon SB, Schlueter J, Ma J et al (2010) Genome sequence of the palaeopolyploid soybean. Nature 463:178–183
Schwachtje J, Minchin PE, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA 103:12935–12940
Shinya T, Hojo Y, Desaki Y, Christeller JT et al (2016) Modulation of plant defense responses to herbivores by simultaneous recognition of different herbivore-associated elicitors in rice. Sci Rep 6:32537
Smith CM, Clement SL (2012) Molecular bases of plant resistance to arthropods. Annu Rev Entomol 57:309–328
Stiglitz J (1997) Reflections on the natural rate hypothesis. J Econ Perspect 11:3–10
Stone BA, Clarke AE (1992) Chemistry and physiology of higher plant 1,3-β-glucans (callose). In: Stone BA, Clarke AE (eds) Chemistry and biology of (1,3)-β-Glucans. La Trobe University Press, Bundoora, pp 365–429
Tamura Y, Hattori M, Yoshioka H, Yoshioka M et al (2014) Map-based cloning and characterization of a brown planthopper resistance gene BPH26 from Oryza sativa L. ssp. indica Cultivar ADR52. Sci Rep 4:5872
Thompson GA, Goggin FL (2006) Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot 57:755–766
Tuelings TC, Ton J (2006) Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr Opin Plant Biol 9:421–427
Ueki S, Citovsky V (2005) Control improves with age: intercellular transport in plant embryos and adults. Proc Natl Acad Sci USA 102:1817–1818
Verma DP, Hong Z (2001) Plant callose synthase complexes. Plant Mol Biol 47:693–701
Volk GM, Franceschi VR (2000) Localization of a calcium channel-like protein in the sieve element plasma membrane. Aust J Plant Physiol 27:779–786
Walling LL (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19:195–216
Wan L, Zha W, Cheng X, Liu C et al (2011) A rice β-1,3-glucanase gene Osg1 is required for callose degradation in pollen development. Planta 233:309–323
Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM (1996) Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J 9:297–304
Wei Z, Hu W, Lin Q, Cheng X et al (2009) Understanding rice plant resistance to the Brown Planthopper (Nilaparvata lugens): a proteomic approach. Proteomics 9:2798–2808
Will T, Tjallingii WF, Thönnessen A, van Bel AJE (2007) Molecular sabotage of plant defense by aphid saliva. Proc Natl Acad Sci USA 104:10536–10541
Will T, Furch ACU, Zimmermann MR (2013) How phloem-feeding insects face the challenge of phloem-located defenses. Front Plant Sci 4:336
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Xie B, Wang X, Zhu M, Zhang Z, Hong Z (2011) CalS7 encodes a callose synthase responsible for callose deposition in the phloem. Plant J 65:1–14
Yamaguchi K, Schinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57:781–803
Zavala JA, Casteel CL, DeLucia EH, Berenbaum MR (2008) Anthropogenic increase in carbon dioxide compromises plant defense against invasive insects. Proc Natl Acad Sci USA 105:5129–5133
Zavala JA, Casteel CL, Nabity PD, Berenbaum MR, DeLucia EH (2009) Role of cysteine proteinase inhibitors in preference of Japanese beetles (Popillia japonica) for soybean (Glycine max) leaves of different ages and grown under elevated CO2. Oecologia 161:35–41
Zavala JA, Nabity PD, Delucia EH (2013) An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu Rev Entomol 58:79–97
Zhang YJ, Wu QJ, Xu BY, Zhu GR (2003) Dangerous alien invasive species–Frankliniella occidentalis occurred in Beijing. Plant Protection 29:58–59
Zheng L, Yamaji N, Yokosho K, Ma JF (2012) YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 24:3767–3782
Acknowledgements
This research was funded by the National Nature Science Foundations of China (NSFC) (31871963, 31272051, 31660546), the Fundamental Research Funds for the Central Universities (KYZ201818), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX180665), and the Qing-Lan Project of Jiangsu Province of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Ethical approval
All applicable international, national and institutional guidelines for the care and use of animals were considered in the present investigation.
Informed consent
All authors of this manuscript accepted that the paper is submitted for publication in the Journal of Pest Science, and reported that this paper has not been published or accepted for publication in another journal, and it is not under consideration at another journal.
Additional information
Communicated by M. Traugott.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue on novel management tactics for the Western Flower Thrips.
Rights and permissions
About this article
Cite this article
Qian, L., Liu, X., Huang, Z. et al. Elevated CO2 enhances the host resistance against the western flower thrips, Frankliniella occidentalis, through increased callose deposition. J Pest Sci 94, 55–68 (2021). https://doi.org/10.1007/s10340-019-01123-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01123-7