Abstract
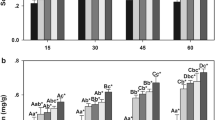
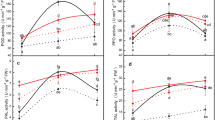
Changes in the activity of some adaptive enzymes of the bird cherry–oat aphid R. padi after transfer from primary (bird cherry) to secondary (triticales) host plants were assessed. The following groups of enzymes were studied: (1) transferases—glutathione S-transferase (GST) and UDP-glucosyltransferase (UDPGT); (2) antioxidant enzymes—superoxide dismutase (SOD) and catalase (CAT); (3) oxidoreductases—polyphenol oxidase (PPO) and peroxidase (PX); and (4) glucoside hydrolases—α- and β-glucosidase. The activity of the transferases and the antioxidant enzymes increased after transfer to the secondary host, but the level of activity was closely associated with feeding duration on the secondary host. The strongest induction was noted for SOD, the activity of which was more than three times greater on the secondary than on the primary host. In contrast, transfer of the bird cherry–oat aphid was accompanied by a decline in activity of PPO, PX, and β-glucosidase; PPO and PX activity was 50% less in aphids fed on the secondary host rather than on the primary host. Activity of α-glucosidase increased after prolonged feeding on the secondary host. The results indicated that the adaptive enzymes of the bird cherry–oat aphid enable it to feed on distantly related host plants.
Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. doi:10.1016/S0076-6879(84)05016-3
Ahmad S (1992) Biochemical defense of pro-oxidant plant allelochemicals by herbivorous insects. Biochem Syst Ecol 20:269–296. doi:10.1016/0305-1978(92)90040-K
Barbehenn RV (2002) Gut-based antioxidant enzymes in a polyphagous and graminivorous grasshopper. J Chem Ecol 28:1329–1347. doi:10.1023/A:1016288201110
Barbehenn RV, Poopat U, Spencer B (2003) Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem Mol Biol 33:125–130. doi:10.1016/S0965-1748(02)00183-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:254–258. doi:10.1016/0003-2697(76)90527-3
Bull DL, Ivie GW, Beier RC, Pryor NW (1986) In vitro metabolism of linear furanocoumarin (8-methoxypsoralen, xanthotoxin) by mixed function oxidases of larvae of black swallowtail butterfly and fall armyworm. J Chem Ecol 12:885. doi:10.1007/BF01020258
Chararas C, Chipoulet JM (1982) Purification by chromatography and properties of a β-glucosidase from the larvae of Phorocantha semipunctata. Comp Biochem Physiol 72B:559–564
Dixon AFG (1971) The life-cycle and host preferences of the bird cherry–oat aphid Rhopalosiphum padi L., and their bearing on the theories of host alternation in aphids. Ann Appl Biol 68:135–147. doi:10.1111/j.1744-7348.1971.tb06450.x
Dixon AFG (1985) Aphid ecology. Blackie, Glasgow, p 157
Eastop VF (1973) Deduction from the present day host plants of aphids and related insects. In: Insects/Plant Relationships Symposium Royal Entomology Society London 6:157–158
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. doi:10.1016/0003-2697(76)90488-7
Ferman H, Dimond AE (1969) Peroxidase activity and Phytophtora resistance in different organes of the potato plant. Phytopathology 57:69–72
Figueroa CC, Koenig C, Araya C, Santos MJ, Niemeyer HM (1999) Effect of DIMBOA, a hydroxamic acid from cereals, on peroxisomal and mitochondrial enzymes from aphids: evidence fort he presence of peroxisomes in aphids. J Chem Ecol 25:2465–2475. doi:10.1023/A:1020870023736
Glinwood RT, Pettersson J (2000) Change in response of Rhopalosiphum padi spring migrants to the repellent winter host component methyl salicylate. Entomol Exp Appl 94:325–330. doi:10.1023/A:1003902321787
Katagiri C (1979) α-d-glucosidase in the serum of the american cockroach Periplaneta americana. Insect Biochem 9:199–204. doi:10.1016/0020-1790(79)90051-9
Krishnan N, Kodrik D (2006) Antioxidant enzymes in Spodoptera littoralis (Boisduval): are they enhanced to protect gut tissues during oxidative stress? J Insect Physiol 52:11–20. doi:10.1016/j.jinsphys.2005.08.009
Laurema S, Varis A-L, Miettinen H (1985) Studies on enzymes in the salivary glands of Lygus rugulipensis (Hemiptera: Miridae). Insect Biochem 15:211–224. doi:10.1016/0020-1790(85)90010-1
Lee K, Berenbaum MR (1990) Defense of parsnip webworm against phototoxic furanocoumarins: role of antioxidant enzymes. J Chem Ecol 16:2451–2460. doi:10.1007/BF01017468
Leszczyński B, Dixon AFG (1992) Resistance of cereal aphids: the interaction between hydroxamic acids and glutathione S-transferases in the grain aphid Sitobion avenae (F.) (Hom., Aphididae). J Appl Entomol 113:61–67
Leszczynski B, Jozwiak B, Laskowska I, Szynkarczyk S (1998) Wiosenne migracje mszycy czeremchowo-zbożowej (Rhopalosiphum padi L.) na pszenżyto (in Polish). Prog Plant Prot/Postępy w Ochronie Roślin 38(2):389–391
Leszczynski B, Jozwiak B, Tjallingii WF, Matok H (1999) Are xenobiotics induce host-plant alternation of bird cherry–oat aphid? Abstracts of 16th Annals Meeting ISCE. Marseille 13–17 P-76
Leszczynski B, Jozwiak B, Urbańska A, Dixon AFG (2003) Does cyanogenesis influence host alternation of the bird cherry–oat aphid? EJPAU 6(1)
Leszczyński B, Matok H, Dixon AFG (1992) Resistance of cereals to aphids: the interaction between hydroxamic acids and UDP-glucose transferases in the grain aphid Sitobion avenae (F.) (Hom., Aphididae). J Chem Ecol 18:1189–1200. doi:10.1007/BF00980073
Lindroth RL (1989) Host plant alteration of detoxication activity in Papilio glaucus glaucus. Entomol Exp Appl 50:29–35. doi:10.1007/BF00190125
Lukasik I (2007) Changes in activity of superoxide dismutase and catalase within cereal aphids in response to plant o-dihydroxyphenols. J Appl Entomol 131:209–214. doi:10.1111/j.1439-0418.2006.01136.x
Łukasik I (2001) Aktywność wybranych enzymów mszycy czeremchowo-zbożowej (Rhopalosiphum padi L.) podczas zmiany roślinnych żywicieli. PhD dissertation. Akademia Podlaska, Siedlce (in Polish)
Łukasik I (2006) Effect of o-dihydroxyphenols on antioxidant defence mechanisms of cereal aphids associated with glutathione. Pestycydy/Pesticides 3(4):67–73
Łukasik I, Jóźwiak B, Leszczyński B, Matok H (2001) Changes in sugar metabolism while host alternation of bird cherry–oat aphid, Rhopalosiphum padi L. Aphids Other Homopterous Insects 8:91–98
Miles PW (1964) Studies on the salivary physiology of plant bugs oxidase activity in the salivary apparatus and saliva. J Insect Physiol 10:121–129. doi:10.1016/0022-1910(64)90100-3
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. doi:10.1016/S1360-1385(02)02312-9
Moloi MJ, van der Westhuizen AJ (2006) The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J Plant Physiol 163:1118–1125. doi:10.1016/j.jplph.2005.07.014
Moran NA (1992) The evolution of aphid life cycle. Annu Rev Entomol 37:321–345. doi:10.1146/annurev.en.37.010192.001541
Morello A, Repetto Y (1979) UDP-glucosyltransferase activity of housefly microsomal fraction. Biochem J 177:809–812
Mukanganyama S, Figueroa CC, Hasler JA, Niemeyer HM (2003) Effects of DIMBOA on detoxification enzymes of the aphid Rhopalosiphum padi (Homoptera: aphididae). J Insect Physiol 49:223–229. doi:10.1016/S0022-1910(02)00269-X
Müller CB, Williams IS, Hardie J (2001) The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol Entomol 26:330–340. doi:10.1046/j.1365-2311.2001.00321.x
Mullin CA (1986) Adaptive divergence of chewing and sucking arthopods to plant allelochemicals. In: Brattsten LB, Ahmad S (eds) Molecular aspects of insect–plant Associations. Plenum Press, New York, pp 175–209
Powell G, Hardie J (2001) The chemical ecology of aphid host alternation: how do return migrants find the primary host plant? Appl Entomol Zool (Jpn) 36:259–267. doi:10.1303/aez.2001.259
Rose RL, Sparks TC, Smith CM (1989) The influence of resistance soybean (PI 227687) foliage and cumestrol on the metabolism of xenobiotics by soybean looper Pseudoplusia includens (Walter). Pestic Biochem Physiol 34:17–26. doi:10.1016/0048-3575(89)90136-3
Sandström J (2000) Nutritional quality of phloem sap in relation to host plant-alternation in the bird cherry–oat aphid. Chemoecology 10:17–24. doi:10.1007/s000490050003
Yu SJ (1982) Host plant induction of glutathione S-transferase in the fall armyworm. Pestic Biochem Physiol 18:1011–1016. doi:10.1016/0048-3575(82)90092-X
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Juen.
Rights and permissions
About this article
Cite this article
Łukasik, I. Effect of host plant alternation on some adaptive enzymes of the bird cherry–oat aphid, Rhopalosiphum padi (L.). J Pest Sci 82, 203–209 (2009). https://doi.org/10.1007/s10340-008-0240-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-008-0240-y