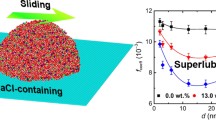
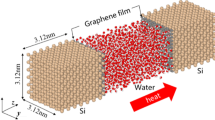
Abstract
Our extensive nonequilibrium and equilibrium molecular dynamics simulations reveal the ion-induced interfacial friction reduction of NaCl-containing water nanoflow over graphene. Even under a normal pressure, with the increase in ion concentration, the friction coefficients of water nanoflow at the water/graphene interfaces decrease, and the slip lengths and shear viscosities of water nanoflow increase. The interfacial friction reduction with the increase in ion concentration is mainly attributed to the decrease in interfacial hydrated ions and the weaker binding of water molecules with graphene at a higher ion concentration. The presence of ions changes the hydrogen bond networks and structural configuration of water molecules close to the underlying graphene sheets and plays a key role in reducing the interfacial friction between water nanoflow and graphene.






Similar content being viewed by others
Data Availability
All data generated or analyzed used to support the findings of this study are included within the article and supplementary material.
References
Bocquet L, Tabeling P. Physics and technological aspects of nanofluidics. Lab Chip. 2014;14:3143–58. https://doi.org/10.1039/c4lc00325j.
van der Heyden FHJ, Bonthuis DJ, Stein D, Meyer C, Dekker C. Electrokinetic energy conversion efficiency in nanofluidic channels. Nano Lett. 2006;6:2232–7. https://doi.org/10.1021/nl061524l.
Pennathur S, Eijkel JCT, van den Berg A. Energy conversion in microsystems: is there a role for micro/nanofluidics? Lab Chip. 2007;7:1234–7. https://doi.org/10.1039/b712893m.
Chen HM, Xu Y, Bai L, Jiang Y, Zhang JS, Zhao C, et al. Crumpled graphene triboelectric nanogenerators: smaller devices with higher output performance. Adv Mater Technol. 2017;2:1700044. https://doi.org/10.1002/admt.201700044.
Chu H, Jang H, Lee Y, Chae Y, Ahn JH. Conformal, graphene-based triboelectric nanogenerator for self-powered wearable electronics. Nano Energy. 2016;27:298–305. https://doi.org/10.1016/j.nanoen.2016.07.009.
Lee JH, Lee KY, Kumar B, Tien NT, Lee NE, Kim SW. Highly sensitive stretchable transparent piezoelectric nanogenerators. Energy Environ Sci. 2013;6:169–75. https://doi.org/10.1039/c2ee23530g.
Abolhasani MM, Shirvanimoghaddam K, Naebe M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos Sci Technol. 2017;138:49–56. https://doi.org/10.1016/j.compscitech.2016.11.017.
Amollo TA, Mola GT, Kirui MSK, Nyamori VO. Graphene for thermoelectric applications: prospects and challenges. Crit Rev Solid State Mater Sci. 2018;43:133–57. https://doi.org/10.1080/10408436.2017.1300871.
Hossain MS, Al-Dirini F, Hossain FM, Skafidas E. High performance graphene nano-ribbon thermoelectric devices by incorporation and dimensional tuning of nanopores. Sci Rep. 2015;5:11297. https://doi.org/10.1038/srep11297.
Chen CY, Hone J. Graphene nanoelectromechanical systems. Proc IEEE. 2013;101:1766–79. https://doi.org/10.1109/JPROC.2013.2253291.
Qian ZY, Liu FZ, Hui Y, Kar S, Rinaldi M. Graphene as a massless electrode for ultrahigh-frequency piezoelectric nanoelectromechanical systems. Nano Lett. 2015;15:4599–604. https://doi.org/10.1021/acs.nanolett.5b01208.
Yin J, Zhang ZH, Li XM, Zhou JX, Guo WL. Harvesting energy from water flow over graphene? Nano Lett. 2012;12:1736–41. https://doi.org/10.1021/nl300636g.
Yin J, Zhang ZH, Li XM, Yu J, Zhou JX, Chen YQ, et al. Waving potential in grapheme. Nat Commun. 2014;5:3582. https://doi.org/10.1038/ncomms4582.
Yin J, Li XM, Yu J, Zhang ZH, Zhou JX, Guo WL. Generating electricity by moving a droplet of ionic liquid along grapheme. Nat Nanotechnol. 2014;9:378–83. https://doi.org/10.1038/NNANO.2014.56.
Kwak SS, Lin SS, Lee JH, Ryu H, Kim TY, Zhong HK, et al. Triboelectrification-induced large electric power generation from a single moving droplet on graphene/polytetrafluoroethylene. ACS Nano. 2016;10:7297–302. https://doi.org/10.1021/acsnano.6b03032.
Lee SH, Kang YB, Jung W, Jung Y, Kim S, Noh H. Flow-induced voltage generation over monolayer graphene in the presence of herringbone grooves. Nanoscale Res Lett. 2013;8:487. https://doi.org/10.1186/1556-276X-8-487.
Zhong HK, Wu ZQ, Li XQ, Xu WL, Xu S, Zhang SJ, et al. Graphene based two dimensional hybrid nanogenerator for concurrently harvesting energy from sunlight and water flow. Carbon. 2016;105:199–204. https://doi.org/10.1016/j.carbon.2016.04.030.
Zhong HK, Xia J, Wang FC, Chen HS, Wu HA, Lin SS. Graphene-piezoelectric material heterostructure for harvesting energy from water flow. Adv Funct Mater. 2017;27:1604226. https://doi.org/10.1002/adfm.201604226.
Xiong W, Liu JZ, Ma M, Xu ZP, Sheridan J, Zheng QS. Strain engineering water transport in graphene nanochannels. Phys Rev E. 2011;84: 056329. https://doi.org/10.1103/PhysRevE.84.056329.
Wagemann E, Misra S, Das S, Mitra SK. Quantifying water friction in misaligned graphene channels under angstrom confinements. ACS Appl Mater Interfaces. 2020;12:35757–64. https://doi.org/10.1021/acsami.0c10445.
Herrero C, Tocci G, Merabia S, Joly L. Fast increase of nanofluidic slip in supercooled water: the key role of dynamics. Nanoscale. 2020;12:20396–403. https://doi.org/10.1039/d0nr06399a.
Li JCA, Zhu YB, Xia J, Fan JC, Wu HA, Wang FC. Anomalously low friction of confined monolayer water with a quadrilateral structure. J Chem Phys. 2021;154: 224508. https://doi.org/10.1063/5.0053361.
Greenwood G, Kim JM, Zheng QL, Nahid SM, Nam S, Espinosa-Marzal RM. Effects of layering and supporting substrate on liquid slip at the single-layer graphene interface. ACS Nano. 2021;15:10095–106. https://doi.org/10.1021/acsnano.1c01884.
Xie YB, Fu L, Niehaus T, Joly L. Liquid–solid slip on charged walls: the dramatic impact of charge distribution. Phys Rev Lett. 2020;125: 014501. https://doi.org/10.1103/PhysRevLett.125.014501.
Raviv U, Giasson S, Kampf N, Gohy JF, Jerome R, Klein J. Lubrication by charged polymers. Nature. 2003;425:163–5. https://doi.org/10.1038/nature01970.
Wang HD, Liu YH, Liu WR, Liu YM, Wang KP, Li JJ, et al. Superlubricity of polyalkylene glycol aqueous solutions enabled by ultrathin layered double hydroxide nanosheets. ACS Appl Mater Interfaces. 2019;11:20249–56. https://doi.org/10.1021/acsami.9b03014.
Ma Q, He T, Khan AM, Wang Q, Chung YW. Achieving macroscale liquid superlubricity using glycerol aqueous solutions. Tribol Int. 2021;160: 107006. https://doi.org/10.1016/j.triboint.2021.107006.
Ge XY, Li JJ, Zhang CH, Liu YH, Luo JB. Superlubricity and antiwear properties of in situ-formed ionic liquids at ceramic interfaces induced by tribochemical reactions. ACS Appl Mater Interfaces. 2019;11:6568–74. https://doi.org/10.1021/acsami.8b21059.
Gao TY, Li JJ, Yi S, Luo JB. Potential-dependent friction on a graphitic surface in ionic solution. J Phys Chem C. 2020;124:23745–51. https://doi.org/10.1021/acs.jpcc.0c07358.
Rosenhek-Goldian I, Kampf N, Klein J. Trapped aqueous films lubricate highly hydrophobic surfaces. ACS Nano. 2018;12:10075–83. https://doi.org/10.1021/acsnano.8b04735.
Diao YJ, Greenwood G, Wang MC, Nam S, Espinosa-Marzal RM. Slippery and sticky graphene in water. ACS Nano. 2019;13:2072–82. https://doi.org/10.1021/acsnano.8b08666.
Han TY, Zhang CH, Li JJ, Yuan SH, Chen XC, Zhang JY, et al. Origins of superlubricity promoted by hydrated multivalent ions. J Phys Chem Lett. 2020;11:184–90. https://doi.org/10.1021/acs.jpclett.9b03098.
Majumder M, Chopra N, Andrews R, Hinds BJ. Nanoscale hydrodynamics-enhanced flow in carbon nanotubes. Nature. 2005;438:44. https://doi.org/10.1038/43844a.
Whitby M, Quirke N. Fluid flow in carbon nanotubes and nanopipes. Nat Nanotechnol. 2007;2:87–94. https://doi.org/10.1038/nnano.2006.175.
Falk K, Sedlmeier F, Joly L, Netz RR, Bocquet L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 2010;10:4067–73. https://doi.org/10.1021/nl1021046.
Joshi RK, Carbone P, Wang FC, Kravets VG, Su Y, Grigorieva IV, et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science. 2014;343:752–4. https://doi.org/10.1126/science.1245711.
Radha B, Esfandiar A, Wang FC, Rooney AP, Gopinadhan K, Keerthi A, et al. Molecular transport through capillaries made with atomic-scale precision. Nature. 2016;538:222–5. https://doi.org/10.1038/nature19363.
Abascal JLF, Vega C. A general purpose model for the condensed phases of water: TIP4P/2005. J Chem Phys. 2005;123: 234505. https://doi.org/10.1063/1.2121687.
Kann ZR, Skinner JL. A scaled-ionic-charge simulation model that reproduces enhanced and suppressed water diffusion in aqueous salt solutions. J Chem Phys. 2014;141: 104507. https://doi.org/10.1063/1.4894500.
Perez-Hernandez G, Schmidt B. Anisotropy of the water–carbon interaction: molecular simulations of water in low-diameter carbon nanotubes. Phys Chem Chem Phys. 2013;15:4995–5006. https://doi.org/10.1039/c3cp44278k.
Hockney RW, Eastwood JW. Computer simulation using particles. Cambridge: CRC Press; 1988. p. 267–304.
Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys. 1977;23:327–41. https://doi.org/10.1016/0021-9991(77)90098-5.
Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J Comput Phys. 1995;117:1–19. https://doi.org/10.1006/jcph.1995.1039.
Nose S. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–9. https://doi.org/10.1063/1.447334.
Hoover WG. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A. 1985;31:1695–7. https://doi.org/10.1103/PhysRevA.31.1695.
Frank MW. Fluid mechanics. New York: McGraw-Hill; 2011. p. 268.
Bocquet L, Barrat JL. Flow boundary conditions from nano- to micro-scales. Soft Matter. 2007;3:685–93. https://doi.org/10.1039/b616490k.
Haynes WM. CRC handbook of chemistry and physics. Boca Raton: CRC Press; 2014.
Guevara-Carrion G, Vrabec J, Hasse H. Prediction of self-diffusion coefficient and shear viscosity of water and its binary mixtures with methanol and ethanol by molecular simulation. J Chem Phys. 2011;134: 074508. https://doi.org/10.1063/1.3515262.
Rajan AG, Strano MS, Blankschtein D. Liquids with lower wettability can exhibit higher friction on hexagonal boron nitride: the intriguing role of solid-liquid electrostatic interactions. Nano Lett. 2019;19:1539–51. https://doi.org/10.1021/acs.nanolett.8b04335.
Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem Bioenergy. 1997;42:153–60. https://doi.org/10.1016/S0302-4598(96)05097-0.
Luzar A, Chandler D. Effect of environment on hydrogen bond dynamics in liquid water. Phys Rev Lett. 1996;76:928–31. https://doi.org/10.1103/PhysRevLett.76.928.
Luzar A, Chandler D. Hydrogen-bond kinetics in liquid water. Nature. 1996;379:55–7. https://doi.org/10.1038/379055a0.
Randall M, Failey CF. The activity coefficient of non-electrolytes in aqueous salt solutions from solubility measurements. The salting-out order of the ions. Chem Rev. 1927;4:285–90. https://doi.org/10.1021/cr60015a004.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (11972186, 11890674, 51921003), the Western Light Project of CAS (xbzg-zdsys-202118), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
YW: Writing—original draft, performed molecular dynamics simulations, formal analysis, contributed to the discussion of the results. YG: Supervision, supervised the whole research, Writing—original draft, formal analysis, contributed to the discussion of the results. WG: Supervised the research, contributed to the discussion of the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Guo, Y. & Guo, W. Ion-Induced Friction Reduction in Water Nanoflow over Graphene. Acta Mech. Solida Sin. 36, 214–220 (2023). https://doi.org/10.1007/s10338-022-00373-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10338-022-00373-w