Abstract
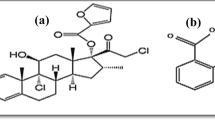
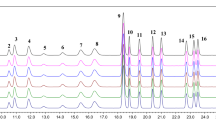
Combination treatment enhances efficacy, minimizes side effects, and improves convenience for patients with diverse treatment schedules. This compilation focuses on the simultaneous estimation of Miconazole nitrate (MNE), Mupirocin (MRN), and Mometasone furoate (MMT) using HPLC and HPTLC in an ointment which is wisely used combination for treatment of skin disorders. The optimized isocratic HPLC method consists of a C18 column, 0.02% triethylamine: methanol (15:85; % v/v), at a flow rate of 0.9 mL/min. The retention times were found to be 4.716 ± 0.082 min for MNE, 13.716 ± 0.188 min for MRN, and 2.241 ± 0.034 min for MMT using the developed method. The method was validated indetail and the quantification range found 20–180 µg/mL for both MNE and MRN and 1–9 µg/mL for MMT. In an alternative HPTLC method, toluene, ethyl acetate, and ethanol (10:3:2; v/v/v) were used as mobile phase on silica gel 60F254-coated aluminum sheets, with scanning at 240 nm. The MNE, MRN and MMT drugs spot eluted at Rf values of 0.65, 0.36, and 0.80, respectively. In-depth validation was performed and assessed statistically, including, specificity, precision, accuracy, reproducibility, and accuracy. The HPTLC method found linear in ranges of 1200–5200 ng/band for MNE and MRN, and 60–260 ng/band for MMT. Consequently, these developed chromatographic methods assessed according to green analytical principle and found sustainable. The developed methods can be effectively applied for the quantitative analysis of commercially available dosage forms.








Similar content being viewed by others
Data Availability
All the data generated or analysed during this study are included in this published article.
References
Ferreira IG, Weber MB, Bonamigo RR (2021) History of dermatology: the study of skin diseases over the centuries. An Bras Dermatol 96:332–345. https://doi.org/10.1016/J.ABD.2020.09.006
Clinical results obtained with a combination of an anti-inflammatory steroid, an antibacterial agent and an antifungal in dermatological outpatient practice - PubMed, (n.d.). https://pubmed.ncbi.nlm.nih.gov/4030166/. Accessed 9 Apr 2024.
Brayfield A (2012) Martindale-The complete drug reference, 38th edn. Pharmaceutical Press (an imprint of RPS publishing), London, UK
Zanwar AS, Sen DB, Maheshwari RA, Chandrakar VR, Seth AK, Sen AK (2020) Simultaneous analysis of mometasone furoate, miconazole nitrate, and nadifloxacin in cream formulation by HPTLC. J Appl Pharm Sci 10:108–115. https://doi.org/10.7324/JAPS.2020.10714
Mupirocin | C26H44O9 | CID 446596 - PubChem, (n.d.). https://pubchem.ncbi.nlm.nih.gov/compound/Mupirocin. Accessed 9 Apr 2024.
Amrutiya N, Bajaj A, Madan M (2009) Development of Microsponges for Topical Delivery of Mupirocin. AAPS PharmSciTech 10:402–409. https://doi.org/10.1208/S12249-009-9220-7
Cavrini V, Di Pietra AM, Gatti R (1989) Analysis of miconazole and econazole in pharmaceutical formulations by derivative UV spectroscopy and liquid chromatography (HPLC). J Pharm Biomed Anal 7:1535–1543. https://doi.org/10.1016/0731-7085(89)80162-1
Akay C, Özkan SA, Şentürk Z, Cevheroğlu A (2002) Simultaneous determination of metronidazole and miconazole in pharmaceutical dosage forms by RP-HPLC. Il Farmaco 57:953–957. https://doi.org/10.1016/S0014-827X(02)01296-X
Erk N, Altun ML (2001) Spectrophotometric resolution of metronidazole and miconazole nitrate in ovules using ratio spectra derivative spectrophotometry and RP-LC. J Pharm Biomed Anal 25:115–122. https://doi.org/10.1016/S0731-7085(00)00485-4
Pagare PK, Satpute CS, Jadhav VM, Kadam V (2012) Forced degradation studies and validated stability-indicating HPTLC method for determination of miconazole nitrate in soft lozenges, Scholars Research Library Der Pharmacia Lettre 4:1793–1804. www.scholarsresearchlibrary.com.
Devaraj S, Nagarajan NC, Sivaperuman A (2020) RP-UPLC method development and validation for simultaneous estimation of mometasone furoate and miconazole nitrate in semisolid dosage form. Acta Pharmaceutica Sciencia 58:335–348. https://doi.org/10.23893/1307-2080.APS.05819
El-Bagary R, Fouad MA, El-Bagary RI, Fouad MA, El-Shaal MA, Tolba EH (2013) Derivative, derivative of the ratio spectrophotometric and stability-indicating RP-HPLC methods for the determination of mometasone furoate and miconazole nitrate in cream, Available Online https://www.jocpr.com/. Journal of Chemical and Pharmaceutical Research 368–378. https://www.researchgate.net/publication/286101404.
Ramzia I, Marwa AF, Manal AE, Enas HT (2013) Derivative, derivative of the ratio spectrophotometric and stability-indicating RP-HPLC methods for the determination of mometasone furoate and miconazole nitrate in cream. J Chem Pharm Res 5:368–378. https://www.jocpr.com/abstract/derivative-derivative-of-the-ratio-spectrophotometric-and-stabilityindicating-rphplc-methods-for-the determination-of-mo-2121.html. Accessed 9 Apr 2024.
De Zan MM, Cámara MS, Robles JC, Kergaravat SV, Goicoechea HC (2009) Development and validation of a simple stability-indicating high performance liquid chromatographic method for the determination of miconazole nitrate in bulk and cream formulations. Talanta 79:762–767. https://doi.org/10.1016/J.TALANTA.2009.04.060
Aboul-Enein HY, Ali I (2002) Comparative study of the enantiomeric resolution of chiral antifungal drugs econazole, miconazole and sulconazole by HPLC on various cellulose chiral columns in normal phase mode. J Pharm Biomed Anal 27:441–446. https://doi.org/10.1016/S0731-7085(01)00575-1
Sivannarayana P, Rambabu K (2016) Simultaneous assay of mupirocin and metronidazole in formulations using reverse phase-high performance liquid chromatography. Int J Bioassays. https://doi.org/10.21746/IJBIO.2016.12.008
Echevarría L, Blanco-Príeto MJ, Campanero MA, Santoyo S, Ygartua P (2003) Development and validation of a liquid chromatographic method for in vitro mupirocin quantification in both skin layers and percutaneous penetration studies. J Chromatogr B Analyt Technol Biomed Life Sci 796:233–241. https://doi.org/10.1016/j.jchromb.2003.07.011
Kalal DJ, Redasani VK (2022) Stability-indicating RP-HPLC method development and validation for estimation of Mupirocin calcium in bulk and in pharmaceutical formulation. Future J Pharm Sci. https://doi.org/10.1186/S43094-022-00412-W
Kale R, Shete P, Doifode D, Chitlange S (2021) Analytical method development and validation for simultaneous determination of simvastatin and mupirocin using reverse-phase high-pressure liquid chromatographic method. Turk J Pharm Sci 18:438. https://doi.org/10.4274/TJPS.GALENOS.2020.58897
Vichare V, Choudhari VP, Reddy MV (2018) Study of intrinsic stability of mometasone furoate in presence of salicylic acid by hptlc and characterization cytotoxicity testing major degradation product mometasone furoate. Curr Pharm Anal 15:592–603. https://doi.org/10.2174/1573412914666180418162143
Sayed RA, El-Masri MM, Hassan WS, El-Mammli MY, Shalaby A (2018) Validated stability-indicating methods for determination of Mometasone Furoate in presence of its alkaline degradation product. J Chromatogr Sci 56:254–261. https://doi.org/10.1093/CHROMSCI/BMX108
Teng XW, Foe K, Brown KF, Cutler DJ, Davies NM (2001) High-performance liquid chromatographic analysis of mometasone furoate and its degradation products: application to in vitro degradation studies. J Pharm Biomed Anal 26:313–319. https://doi.org/10.1016/S0731-7085(01)00408-3
Shaikh S, Muneera MS, Thusleem OA, Tahir M, Kondaguli AV (2009) A simple RP-HPLC method for the simultaneous quantitation of chlorocresol, mometasone furoate, and fusidic acid in creams. J Chromatogr Sci 47:178–183. https://doi.org/10.1093/CHROMSCI/47.2.178
Patel B, Patel S (2023) A specific high-performance thin-layer chromatography method validated for estimation of mometasone furoate and olopatadine hydrochloride. Sep Sci Plus 6:2300032. https://doi.org/10.1002/SSCP.202300032
Srinivasarao K, Gorule V, Ch VR, Krishna V (2012) Validated method development for estimation of Formoterol Fumarate and Mometasone Furoate in metered dose inhalation form by high performance liquid chromatography. J Anal Bioanal Tech. https://doi.org/10.4172/2155-9872.1000153
Shaikh KA, Patil AT (2013) Stability-indicating HPLC method for the determination of Mometasone Furoate, oxymetazoline, phenyl ethanol and benzalkonium chloride in nasal spray solution. J Trace Anal Food Drugs. https://doi.org/10.7726/JTAFD.2013.1002
Roy C, Chakrabarty J (2013) Stability-indicating validated novel rp-hplc method for simultaneous estimation of methylparaben, ketoconazole, and Mometasone Furoate in topical pharmaceutical dosage formulation, ISRN. Anal Chem 2013:1–9. https://doi.org/10.1155/2013/342794
Sharma N, Rao S, Vaghela B (2013) Validated stability-indicating high-performance liquid chromatographic method for estimation of degradation behaviour of Eberconazole Nitrate and Mometasone Furoate in cream formulation. Indian J Pharm Sci 75:76. https://doi.org/10.4103/0250-474X.113530
Jahani M, Akaberi M, Heidari T, Kamali H, Nejabat M, Rajabi O, Hadizadeh F (2023) Simultaneous determination of mometasone furoate and calcipotriol in a binary mixture by validated HPLC and chemometric-assisted UV spectrophotometric methods and identification of degradation products by LC-MS, Iran J Basic. Med Sci 26:37–47. https://doi.org/10.22038/IJBMS.2022.65436.14396
International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use ICH Harmonized Tripartite Guideline Validation Of Analytical Procedures: Text And Methodology Q2(R1), N.D.
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/J.TALANTA.2018.01.013
Płotka-Wasylka J, Wojnowski W (2021) Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem 23:8657–8665. https://doi.org/10.1039/D1GC02318G
Kammoun AK, Khayat MT, Almalki AJ, Youssef RM (2023) Development of validated methods for the simultaneous quantification of Finasteride and Tadalafil in newly launched FDA-approved therapeutic combination: greenness assessment using AGP, analytical eco-scale, and GAPI tools. RSC Adv 13:11817–11825. https://doi.org/10.1039/d3ra01437a
Bherje S, Patel M (2024) A green perspective on simultaneous HPLC and UV spectrophotometric estimation of Udenafil and Dapoxetine hydrochloride in pharmaceutical formulations. Green Anal Chem 9:100106. https://doi.org/10.1016/J.GREEAC.2024.100106
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE—Analytical GREEnness metric approach and software. Anal Chem. https://doi.org/10.1021/ACS.ANALCHEM.0C01887
Pena-Pereira F, Tobiszewski M, Wojnowski W, Psillakis E (2022) A Tutorial on AGREEprep an analytical greenness metric for sample preparation. Adv Sample Prep 3:100025. https://doi.org/10.1016/J.SAMPRE.2022.100025
Nahata A, Patel M, Krishna Muchakayala S (2024) Strategic approaches to elevate quality and sustainability in drug development: comprehensive pretomanid (PA-824) chemical stability study using QbD and green chemistry principles. Microchem J 200:110413
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical Eco-Scale for assessing the greenness of analytical procedures, TrAC -. Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Aziza N, Khaydarov K, Zafar M, Alsakkaf WAA, Alkahtani J, Ahmad M, Makhkamov T, Djumayeva Z, Zengin G, Eshboyevich TK, Beilerli A, Gareev I, Ochilov U, Sultanovich IB, Iskandarovna UZ, Wibawa IPAH (2024) Chromatographic authentication of botanical origin: Herbaceous pollen profiling with HPLC, HPTLC and GC–MS analysis. Biomed Chromatogr. https://doi.org/10.1002/bmc.5852
Galal MM, Abdullah SA, Mohamed OY, Moustafa AA (2023) Greenness assessment of two chromatographic methods developed for the determination of Mupirocin in two binary mixtures along with its impurity. BMC Chem. https://doi.org/10.1186/s13065-023-01055-5
Funding
The authors have not received any funds for the work.
Author information
Authors and Affiliations
Contributions
Aarti Sachin Zanwar, Ashim Kumar Sen, Dhanya B Sen, Sachin Zanwar: Writing – original draft, Material preparation, data collection and analysis, Validation, Investigation, Formal analysis. Anuj N. Nahata: Writing – original draft, GREEnness Assessment. Mital Patel: Review and final editing, supervision, project administration, conceptualization.
Corresponding author
Ethics declarations
Conflict of Interest
All the authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanwar, A.S., Nahata, A.N., Sen, A.K. et al. Comprehensive Quantification of Miconazole Nitrate, Mupirocin, and Mometasone Furoate: a Dual Analysis via HPLC and HPTLC with Comparative Evaluation Against Greenness Parameters. Chromatographia (2024). https://doi.org/10.1007/s10337-024-04338-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10337-024-04338-8