Abstract
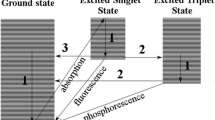
Dibenzoazecines are a new class of potential neuroleptics with a high potential for the treatment of schizophrenia. Initial stress tests indicated that the lead compound, LE404, decomposes when exposed to oxygen and sunlight. In this follow-up study, the influence of oxidative stress and photosensitivity was investigated in accordance with the ICH guidelines. These studies are of great importance for new APIs, as phototoxic and photoallergic reactions pose a significant risk to patients, especially with long-term medication, as it is not possible to completely avoid exposure to sunlight. Furthermore, the identification and prediction of the chemical lability of LE404 can be used to determine suitable storage conditions to prevent compound degradation. The effects of exposure of LE404 to a light source similar in spectrum and intensity to natural sunlight were investigated according to ICH-Q1B. Furthermore, the influence of oxidizing agents was investigated under exclusion of light. Two degradation products were identified. The extent and rate of degradation were continuously monitored using RP-HPLC–UV. Chromatographic separations were performed with a phenomenex™ Gemini 5 µm C18 (250 × 4.60 mm) column and acetonitrile/KH2PO4 buffer (4 mmol L−1, pH 2.5) as mobile phase at 220 nm. The photodegradation product was isolated using semi-preparative RP-HPLC. The oxidation product was obtained by quantitative conversion of LE404 in hydrogen peroxide and subsequent purification by preparative TLC. The structures of both degradation products were elucidated using HR-MS/MS, 1D- and 2D-NMR as well as FT-IR spectroscopy. The characterization of the degradation products serves as the basis for subsequent investigations into their toxicity.






Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Lehmann J, Mohr P, Schweikert PM, Decker M, Hoefgen B/Assignee: Friedrich-Schiller-Universität Jena, Germany. Patent: DE 10 2005 025 625 A1 2006.12.07
Bonnot O, Dumas N (2014) Schizophrenic disorders in adolescence. Rev Prat 64:499–504
Jauhar S, Johnstone M, McKenna PJ (2020) Schizophrenia Lancet 399:473–486
Goff DC (2021) The pharmacologic treatment of schizophrenia—2021. JAMA 325:175–176
Hoefgen B, Decker M, Mohr P, Schramm AM, Rostom SAF, El-Subbagh H, Schweikert PM, Rudolf DR, Kassack MU, Lehmann J (2006) Dopamine/Serotonin receptor ligands. 10:1 SAR studies on azecine-type dopamine receptor ligands by functional screening at human cloned D1, D2L, and D5 receptors with a microplate reader based calcium assay lead to a novel potent D1/D5 selective antagonist. J Med Chem 49:760–769
Mohr P, Decker M, Enzensperger C, Lehmann J (2006) Dopamine/Serotonin receptor ligands. 12(1): SAR studies on Hexahydro-dibenz[d, g]azecines lead to 4-Chloro-7-methyl-5,6,7,8,9,14-hexahydrodibenz[d, g]azecin-3-ol, the First Picomolar D5-selective dopamine-receptor antagonist. J Med Chem 49:2110–2116
Schulze M, Siol O, Robaa D, Mueller FKU, Enzensperger C, Fleck C, Lehmann J (2012) Molecular combination of the dopamine and serotonin scaff olds yield in novel antipsychotic drug candidates—characterization by in vivo experiments. Drug Res 62:252–260
Zergiebel S, Fleck C, Arndt HD, Enzensperger C, Seeling A (2017) Synthesis and characterization of new azecine-derivatives as potential neuroleptics. Drug Res 67:466–475
Zergiebel S, Arndt HD, Seeling A (2017) Optimized synthesis of new LE404-derived azecine-prodrugs. Tetrahedron Lett 58:3640–3642
Zergiebel S, Seeling A (2018) In vitro studies on physiological and chemical stability of new LE404-derivatives with extended half-life. Drug Res 68:514–520
Lugović-Mihić L, Duvančić T, Ferček I, Vuković P, Japundžić I, Ćesić D (2017) Drug-induced photosensitivity—a continuing diagnostic challenge. Acta Clin Croat 56:277–283
Fachinformation (Zusammenfassung der Merkmale des Arzneimittels) Doxycyclin STADA® 100 mg Filmtabletten, Doxycyclin STADA® 200 mg Filmtabletten, STADApharm. http://fachinformation.srz.de/pdf/stadapharm/doxycyclinstada100mg-200mgfilmtabletten.pdf. Accessed 2 Jun 2021
ICH guideline (2006) Impurities in new drug substances Q3A(R2); https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf. Accessed 30 Jan 2023
ICH guideline (2006) Impurities in new drug products Q3B(R2); https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf. Accessed 30 Jan 2023
Zergiebel S, Seeling A (2021) Robust and fast UV–HPLC method for biotransformation analysis of azecines. Chromatographia 84:275–283
ICH Guideline (1998) Topic Q1B photostability testing of new active substances and medicinal products. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-b-photostability-testing-new-active-substances-and-medicinal-products-step-5_en.pdf. Accessed 30 Jan 2023
ICH Guideline (2003) Stability testing of new drug substances and products; https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf. Accessed 23 Nov 2020
Laus G (2001) Kinetics of acetonitrile-assisted oxidation of tertiary amines by hydrogen peroxide. J Chem Soc Perkin Trans 2:864–868
Brauer HD, Eilers B, Lange A (2002) Formation of singlet molecular oxygen by the Radziszewski reaction between acetonitrile and hydrogen peroxide in the absence and presence of ketones. J Chem Soc Perkin Trans 2:1288–1295
Potmischil F, Duddeck H, Nicolescu A, Deleanu C (2007) Saturated amine oxides: Part 8†. Hydroacridines: Part 27‡. Effects of N-oxidation and of N-quaternization on the 15N NMR chemical shifts of N-methylpiperidine-derived mono-, bi-, and tricycloaliphatic tertiary amines. Magn Reson Chem 45:231–235
Schulze-Sünninghausen D, Becker J, Koos MRM, Luya B (2017) Improvements, extensions, and practical aspects of rapid ASAP-HSQC and ALSOFAST-HSQC pulse sequences for studying small molecules at natural abundance. J Magn Reson 281:151–161
ChemDraw© Professional (2022) PerkinElmer Informatics Inc
Galano A, Pérez-González A, Castañeda-Arriaga R, Muñoz-Rugeles L, Mendoza-Sarmiento G, Romero-Silva A, Ibarra-Escutia A, Rebollar-Zepeda AM, León-Carmona JR, Hernández-Olivares MA, Alvarez-Idaboy JR (2016) Empirically fitted parameters for calculating pKa values with small deviations from experiments using a simple computational strategy. J Chem Inf Model 56:1714–1724
Ripin DH, Evan DA. Evan’s pKA table; http://ccc.chem.pitt.edu/wipf/MechOMs/evans_pKa_table.pdf. Accessed 19 Oct 2023
Integrated Spectral Data Base System of Organic Compounds. National Institute of Advanced Industrial Science and Technology (Japan) CAS: 628–41–1. https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=559. Accessed 20 Oct 2023
Integrated Spectral Data Base System of Organic Compounds, National Institute of Advanced Industrial Science and Technology (Japan) p-Benzoquinone. https://scifinder-n.cas.org/searchDetail/substance/6532ae74b98f6b678c5acf6e/substanceSpectra. Accessed 20 Oct 2023
Integrated Spectral Data Base System of Organic Compounds, National Institute of Advanced Industrial Science and Technology (Japan) (2-methoxy-5-methyl-p-benzoquinone). https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=25937. Accessed 20 Oct 2023
Integrated Spectral Data Base System of Organic Compounds, National Institute of Advanced Industrial Science and Technology (Japan) CAS: 14790-04-6. https://sdbs.db.aist.go.jp/sdbs/cgi-bin/cre_frame_disp.cgi?spectrum_type=ir&sdbsno=27517. Accessed 20 Oct 2023
Mohammed SJ, Salih AK, Rashid MAM, Omer KM, Abdalkarim KA (2020) Synthesis, spectroscopic studies and keto-enol tautomerism of novel 1,3,4-thiadiazole derivative containing 3-Mercaptobutan-2-one and Quinazolin-4-one moieties. Molecules 25:5441
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. Conceptualization: S.Z.; Methodology: S.Z.; Formal analysis and investigation: S.Z., N.U.; Visualization: N.U., S.Z.; Writing - original draft preparation: S.Z.; Writing - review and editing: S.Z., A.S., N.U.; Resources: J.P.; Supervision: A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zergiebel, S., Ueberschaar, N., Plentz, J. et al. HPLC–UV Monitored Photostability-Test of LE404 and Identification of the Degradation Products via NMR and LC–HRMS. Chromatographia 87, 339–349 (2024). https://doi.org/10.1007/s10337-024-04323-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-024-04323-1