Abstract
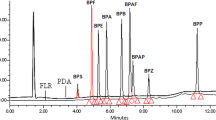
In this study, the efficiency of two detectors—variable wavelength detector (VWD) and fluorescence detector (FLD)—in a liquid chromatograph to quantify azoxystrobin in water was compared. For this, a method was developed in a HPLC-VWD-FLD system using extraction by modified QuEChERS. The detection of azoxystrobin by fluorescence was possible using only a solvent effect, considering that azoxystrobin does not naturally fluoresce. Analytical curves were constructed using matrix-matched calibration, where a low matrix effect was verified in both detectors. In the linearity analysis of the analytical curves, the values of coefficient of determination found for the detectors were 0.9989 and 0.9983 for VWD and FLD, respectively. The relative standard error for the VWD detector was 6.96%, while for the fluorescence detector, it was 5.49%. The limit of detection (LOD) and the limit of quantification (LOQ) were, respectively, 0.33 and 0.68 mg L−1 (VWD) and 0.18 and 0.37 mg L−1 (FLD). The QuEChERS method, originally developed for the extraction of pesticides in food, was adapted for the extraction of azoxystrobin in water, demonstrating efficiency in the process, with precision values lower than 20% and accuracy between 83.58 and 112.88%, within the criteria established by MAPA and Document Nº SANTE/11312/2021. Therefore, it is concluded that both detectors are suitable for the analysis of azoxystrobin in water; however, there is a greater sensitivity for the fluorescence detector, since lower detection and quantification limit values were observed, in addition to a lower standard relative error (RSE).



Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Brondi SHG, de Macedo AN, Vicente GHL, Nogueira ARA (2011) Evaluation of the QuEChERS method and gas chromatography-mass spectrometry for the analysis pesticide residues in water and sediment. Bull Environ Contam Toxicol 86:18–22. https://doi.org/10.1007/s00128-010-0176-9
Somashekar KM, Mahima MR, Manjunath KC (2015) Contamination of water sources in Mysore city by pesticide residues and plasticizer—a cause of health concern. Aquatic Procedia 4:1181–1188. https://doi.org/10.1016/j.aqpro.2015.02.150
Balkan T, Yılmaz Ö (2022) Method validation, residue and risk assessment of 260 pesticides in some leafy vegetables using liquid chromatography coupled to tandem mass spectrometry. Food Chem 384:132516. https://doi.org/10.1016/j.foodchem.2022.132516
Shao Y, Wang M, Cao J et al (2023) A method for the rapid determination of pesticides coupling thin-layer chromatography and enzyme inhibition principles. Food Chem 416:135822. https://doi.org/10.1016/j.foodchem.2023.135822
de Saath KC, O, Fachinello AL, (2018) Crescimento da Demanda Mundial de Alimentos e Restrições do Fator Terra no Brasil. Rev Econ Sociol Rural 56:195–212. https://doi.org/10.1590/1234-56781806-94790560201
ANVISA (2019) Programa de Análise de Resíduos de Agrotóxicos em Alimentos—PARA: Relatório das Amostras Analisadas no período de 2017–2018. Brasília
Gomes HDO, Menezes JMC, da Costa JGM et al (2020) A socio-environmental perspective on pesticide use and food production. Ecotoxicol Environ Safety 197:110627. https://doi.org/10.1016/j.ecoenv.2020.110627
Wang M, Zhao L, Niu Y et al (2023) Magnetic deep eutectic solvent-based dispersive liquid–liquid microextraction for determination of strobilurin fungicides in water, juice, and vinegar by high-performance liquid chromatography. Food Chem X. 18:100711. https://doi.org/10.1016/j.fochx.2023.100711
Wang S, Kong C, Wu N et al (2022) H-beta zeolite-based dispersive solid-phase strategy for the multi-residue determination of pesticides. Anal Chim Acta 1227:340327. https://doi.org/10.1016/j.aca.2022.340327
Brasil (2021) Portaria GM/MS No 888, de 4 de maio de 2021. Ministério da Saúde
World Health Organization (2021) A global overview of national regulations and standards for drinking-water quality, 2nd edn. World Health Organization, Geneva
Lu C, Hou K, Zhou T et al (2023) Characterization of the responses of soil micro-organisms to azoxystrobin and the residue dynamics of azoxystrobin in wheat–corn rotation fields over two years. Chemosphere 318:137918. https://doi.org/10.1016/j.chemosphere.2023.137918
Marczewska P, Płonka M, Rolnik J, Sajewicz M (2020) Determination of azoxystrobin and its impurity in pesticide formulations by liquid chromatography. J Environ Sci Health B 55:599–603. https://doi.org/10.1080/03601234.2020.1746572
Noegrohati S, Hernadi E, Asviastuti S (2018) Matrix effect evaluation and method validation of azoxystrobin and difenoconazole residues in red flesh dragon fruit (Hylocereus polyrhizus) matrices using QuEChERS sample preparation methods followed by LC–MS/MS determination. Bull Environ Contam Toxicol 100:821–826. https://doi.org/10.1007/s00128-018-2317-5
Muhammad N, Zia-ul-Haq M, Ali A et al (2021) Ion chromatography coupled with fluorescence/UV detector: a comprehensive review of its applications in pesticides and pharmaceutical drug analysis. Arab J Chem 14:102972. https://doi.org/10.1016/j.arabjc.2020.102972
Lin G, Ji R, Yao H et al (2020) Fluorescence detection of multiple kinds of pesticides with multi hidden layers neural network algorithm. Optik 211:164632. https://doi.org/10.1016/j.ijleo.2020.164632
Yuan Y-Y, Wang S-T, Liu S-Y et al (2020) Green approach for simultaneous determination of multi-pesticide residue in environmental water samples using excitation-emission matrix fluorescence and multivariate calibration. Spectrochim Acta Part A Mol Biomol Spectrosc 228:117801. https://doi.org/10.1016/j.saa.2019.117801
Chen M, Zhao Z, Lan X et al (2015) Determination of carbendazim and metiram pesticides residues in reapeseed and peanut oils by fluorescence spectrophotometry. Measurement 73:313–317. https://doi.org/10.1016/j.measurement.2015.05.006
Almeida J, Macedo R, Da Cunha A et al (2023) Determination of trifloxystrobin in soy grape juice and natural water by photo-induced fluorescence and high-performance liquid chromatography. J Braz Chem Soc. https://doi.org/10.21577/0103-5053.20230080
Flores JL, Díaz AM, Fernández De Córdova ML (2007) Determination of azoxystrobin residues in grapes, musts and wines with a multicommuted flow-through optosensor implemented with photochemically induced fluorescence. Anal Chim Acta 585:185–191. https://doi.org/10.1016/j.aca.2006.11.076
Guo X, Wang K, Chen G-H et al (2017) Determination of strobilurin fungicide residues in fruits and vegetables by nonaqueous micellar electrokinetic capillary chromatography with indirect laser-induced fluorescence: CE and CEC. Electrophoresis 38:2004–2010. https://doi.org/10.1002/elps.201700060
MAPA (2011) Manual de garantia da qualidade analítica. MAPA/ACS, Brasília
EC (2021) Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021
Anastassiades M, Lehotay SJ (2003) Fast and easy multiresidue method employing acetonitile extraction_partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOCA Int 86:412–431
Abdel Ghani SB, Hanafi AH (2016) QuEChERS method combined with GC-MS for pesticide residues determination in water. J Anal Chem 71:508–512. https://doi.org/10.1134/S1061934816050117
Danzer K, Currie LA (1998) Guidelines for calibration in analytical chemistry. Part I. Fundamentals and single component calibration (IUPAC Recommendations 1998). Pure Appl Chem 70:993–1014. https://doi.org/10.1351/pac199870040993
Barbosa PGA, Martins FICC, Lima LK et al (2018) Statistical analysis for quality adjustment of the analytical curve for determination of pesticide multiresidue in pineapple samples. Food Anal Methods 11:466–478. https://doi.org/10.1007/s12161-017-1017-9
Bazilio FS, Bomfim MVJ, Almeida RJ, Abrantes SMP (2014) Intralaboratory validation of an analytical method for determining the migration of bis(2-ethylhexyl) adipate from packaging to fat foods. Accred Qual Assur 19:195–204. https://doi.org/10.1007/s00769-014-1055-6
de Gomes HO, da Cardoso RS, da Costa JGM et al (2021) Statistical evaluation of analytical curves for quantification of pesticides in bananas. Food Chem 345:128768. https://doi.org/10.1016/j.foodchem.2020.128768
Coly A (1994) Fluorimetric determination of aromatic pesticides in technical formulations. Effects of solvent and of ultraviolet photolysis. Talanta 41:1475–1480. https://doi.org/10.1016/0039-9140(94)E0021-I
Coly A, Aaron J-J (1998) Fluorimetric analysis of pesticides: methods, recent developments and applications. Talanta 46:815–843. https://doi.org/10.1016/S0039-9140(97)00366-4
Hassoon S, Schechter I (2000) In situ fluorimetric determination of pesticides on vegetables. Anal Chim Acta 405:9–15. https://doi.org/10.1016/S0003-2670(99)00747-3
Lawrence JF, Frei RW (1974) Fluorimetric derivatization for pesticide residue analysis. J Chromatogr A 98:253–270. https://doi.org/10.1016/S0021-9673(00)84786-X
Muhammad N, Hussian I, Ali A et al (2022) A comprehensive review of liquid chromatography hyphenated to post-column photoinduced fluorescence detection system for determination of analytes. Arab J Chem 15:104091. https://doi.org/10.1016/j.arabjc.2022.104091
Girame R, Shabeer TPA, Ghosh B et al (2022) Multi-residue method validation and safety evaluation of pesticide residues in seed spices cumin (Cuminum cyminum) and coriander (Coriandrum sativum) by gas chromatography tandem mass spectrometry (GC–MS/MS). Food Chem 374:131782. https://doi.org/10.1016/j.foodchem.2021.131782
WHO (2017) Guidelines for drinking-water quality. World Health Organization, Geneva
Pano-Farias NS, Ceballos-Magaña SG, Muñiz-Valencia R, Gonzalez J (2017) Validation and assessment of matrix effect and uncertainty of a gas chromatography coupled to mass spectrometry method for pesticides in papaya and avocado samples. J Food Drug Anal 25:501–509. https://doi.org/10.1016/j.jfda.2016.09.005
Ferreira JA, Ferreira JMS, Talamini V et al (2016) Determination of pesticides in coconut ( Cocos nucifera Linn.) water and pulp using modified QuEChERS and LC–MS/MS. Food Chem 213:616–624. https://doi.org/10.1016/j.foodchem.2016.06.114
Guedes JAC, Silva RDO, Lima CG et al (2016) Matrix effect in guava multiresidue analysis by QuEChERS method and gas chromatography coupled to quadrupole mass spectrometry. Food Chem 199:380–386. https://doi.org/10.1016/j.foodchem.2015.12.007
Prestes O, Friggi C, Adaime M, Zanella R (2009) QuEChERS - Um método moderno de preparo de amostra para determinação multirresíduo de pesticidas em alimentos por métodos cromatográficos acoplados à espectrometria de massas. Quim Nova 32:1620–1634. https://doi.org/10.1590/S0100-40422009000600046
Acknowledgements
The authors are grateful to the Fundação Cearense de Apoio ao Desenvolvimento—BPI/FUNCAP (Grant number BP4-00172-00105.01.00/20) for financial support of this research.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
HdOG: conceptualization, methodology, formal analysis, investigation, writing—original draft, writing—review and editing. ECLdSB: writing—original draft, investigation. CRFdS: formal analysis, investigation. RdSC: methodology, formal analysis. CdOdS: formal analysis, investigation. LCCdO: resources, data curation. JGMdC: resources, visualization, supervision. RFdN: writing—review and editing, validation, project administration. RNPT: conceptualization, supervision, writing—review and editing, visualization, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval
Ethics approval was not required for this study.
Informed Consent
Informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Gomes, H., da Silva Bento, E.C.L., dos Santos, C.R.F. et al. Comparative Study Between VWD and FLD Detector in HPLC System for Azoxystrobin Quantification in Water. Chromatographia 86, 605–615 (2023). https://doi.org/10.1007/s10337-023-04274-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04274-z