Abstract
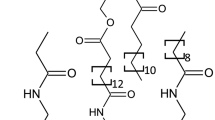
Immobilized artificial membrane chromatography has been reported providing a rough estimation of phospholipidosis induction risk of drugs. Unfortunately, however, the accurate assessment of basic drugs remains a challenge. In this study, we aimed to evaluate the hydrophobic interactions and ionic/polar interactions between a basic drug and an immobilized artificial membrane to clearly discriminate between phospholipidosis inducers and non-inducers. The retention of 14 model basic drugs was determined using mobile phases with varying acetonitrile contents. The Van’t Hoff plot using an acetonitrile-rich mobile phase revealed that most of the inducers showed biphasic retention, whereas the retention of non-inducers was monophasic. Then, effect of the ionic/polar interactions on the discrimination between DIPL inducers and non-inducers was then studied. For this aim, salts concentration was increased in the acetonitrile-rich mobile phase. No significant difference was observed between chlorpromazine (inducer) and pindolol (non-inducer) as the model drugs. We then compared the acetonitrile concentration when the dominant interaction was shifted from the reversed phase retention to the ionic/polar interactions. We found that the acetonitrile levels when the dominant interaction of the inducers shifted from the hydrophobic interaction to the ionic/polar interactions were higher than those of non-induces. In addition, at the optimized acetonitrile content, the inducers and non-inducers can be correctly discriminated. Shift of the dominant interaction in retention to the immobilized artificial membrane shows a possibility as a novel tool for the risk prediction of phospholipidogenisity.






Similar content being viewed by others
Data availability statement
The data that supports the findings of this study are available within the article and its supplementary material.
References
Grumetto L, Russo G, Barbato F (2016) Polar interactions drug/phospholipids estimated by IAM-HPLC vs cultured cell line passage data: their relationships and comparison of their effectiveness in predicting drug human intestinal absorption. Int J Pharm 500:275–290. https://doi.org/10.1016/j.ijpharm.2016.01.019
Grumetto L, Carpentiero C, Barbato F (2012) Lipophilic and electrostatic forces encoded in IAM-HPLC indexes of basic drugs: their role in membrane partition and their relationships with BBB passage data. Eur J Pharm Sci 45:685–692. https://doi.org/10.1016/j.ejps.2012.01.008
Grumetto L, Russo G, Barbato F (2016) Immobilized artificial membrane HPLC derived parameters vs PAMPA-BBB data in estimating in situ measured blood-brain barrier permeation of drugs. Mol Pharmaceutics 13:2808–2816. https://doi.org/10.1021/acs.molpharmaceut.6b00397
Valko KL, Teague SP, Pidgeon C (2017) In vitro membrane binding and protein binding (IAM MB/PB technology) to estimate in vivo distribution: applications in early drug discovery. ADMET DMPK 5:14–38. https://doi.org/10.5599/admet.5.1.373
Ong S, Liu H, Pidgeon C (1996) Immobilized-artificial-membrane chromatography: measurements of membrane partition coefficient and predicting drug membrane permeability. J Chromatogr A 728:113–128. https://doi.org/10.1016/0021-9673(95)00837-3
Vrakas D, Giaginis C, Tsantili-Kakoulidou A (2008) Electrostatic interactions and ionization effect in immobilized artificial membrane retention: a comparative study with octanol-water partitioning. J Chromatogr A 1187:67–78. https://doi.org/10.1016/j.chroma.2008.01.079
Tsopelas F, Stergiopoulos C, Tsakanika LA, Ochsenkühn-Petropoulou M, Tsantili-Kakoulidou A (2017) The use of immobilized artificial membrane chromatography to predict bioconcentration of pharmaceutical compounds. Ecotoxicol Environ Saf 139:150–157. https://doi.org/10.1016/j.ecoenv.2017.01.028
Taillardat-Bertschinger A, Martinet CAM, Carrupt PA, Reist M, Caron G, Fruttero R, Testa B (2002) Molecular factors influencing retention on immobilized artificial membranes (IAM) compared to partitioning in liposomes and n-octanol. Pharm Res 19:729–737. https://doi.org/10.1023/A:1016156927420
Liu X, Testa B, Fahr A (2011) Lipophilicity and its relationship with passive drug permeation. Pharm Res 28:962–977. https://doi.org/10.1007/s11095-010-0303-7
Kodavanti UP, Mehendale HM (1990) Cationic amphiphilic drugs and phospholipid storage disorder. Pharmacol Rev 42(4):327–354
Zhao XL, Chen WJ, Liu ZH, Guo JL, Zhou ZY, Crommen J, Moaddel R, Jiang ZJ (2014) A novel mixed phospholipid functionalized monolithic column for early screening of drug induced phospholipidosis risk. J Chromatogr A 1367:99–108. https://doi.org/10.1016/j.chroma.2014.09.048
Jiang Z, Reilly J (2012) Chromatography approaches for early screening of the phospholipidosis-inducing potential of pharmaceuticals. J Pharm Biomed Anal 61:184–190. https://doi.org/10.1016/j.jpba.2011.11.033
Tomizawa K, Sugano K, Yamada H, Horii I (2006) Physicochemical and cell-based approach for early screening of phospholipidosis-inducing potential. J Toxicol Sci 31:315–324. https://doi.org/10.2131/jts.31.315
Ploemen JHTM, Kelder J, Hafmans T, van de Sandt H, van Burgsteden JA, Salemink PJM, van Esch E (2004) Use of physicochemical calculation of pKa and CLogP to predict phospholipidosis-inducing potential: a case study with structurally related piperazines. Exp Toxic Pathol 55:347–355. https://doi.org/10.1078/0940-2993-00338
van de Water FM, Having J, Ravesloot WT, Horbach GJMJ, Schoonen WGEJ (2011) High content screening analysis of phospholipidosis: validation of a 96-well assay with CHO-K1 and HepG2 cells for the prediction of in vivo based phospholipidosis. Toxicol In Vitro 25:1870–1882. https://doi.org/10.1016/j.tiv.2011.05.026
Iwakuma Y, Okamoto H, Hamaguchi R, Kuroda Y (2019) The limited contribution of the analyte partition to the water-rich layer in immobilized artificial membrane chromatography with an acetonitrile-rich binary mobile phase. Chromatographia 82:1311–1320. https://doi.org/10.1007/s10337-019-03750-9
Alpert AJ (1990) Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr A 499:177–196. https://doi.org/10.1016/S0021-9673(00)96972-3
Guo Y (2015) Recent progress in the fundamental understanding of hydrophilic interaction chromatography (HILIC). Analyst 140:6452–6466. https://doi.org/10.1039/C5AN00670H
Valkó K, Bevan C, Reynolds D (1997) Chromatographic hydrophobicity index by fast-gradient RP-HPLC: a high-throughput alternative to log P/log D. Anal Chem 69:2022–2029. https://doi.org/10.1021/ac961242d
Ong S, Pidgeon C (1995) Thermodynamics of solute partitioning into immobilized artificial membranes. Anal Chem 67:2119–2128. https://doi.org/10.1021/ac00109a034
Valkó K, Du CM, Bevan CD, Reynolds DP, Abraham MH (2000) Rapid-gradient HPLC method for measuring drug interactions with immobilized artificial membrane: comparison with other lipophilicity measures. J Pharm Sci 89:1085–1096. https://doi.org/10.1002/1520-6017(200008)89:8%3C1085::AID-JPS13%3E3.0.CO;2-N
Valkó KL (2016) Lipophilicity and biomimetic properties measured by HPLC to support drug discovery. J Pharm Biomed Anal 130:35–54. https://doi.org/10.1016/j.jpba.2016.04.009
Casartelli A, Bonato M, Cristofori P, Crivellente F, Dal Negro G, Masotto I, Mutinelli C, Valkó K, Bonfante V (2003) A cell-based approach for the early assessment of the phospholipidogenic potential in pharmaceutical research and drug development. Cell Biol Toxicol 19:161–176. https://doi.org/10.1023/A:1024778329320
Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R (1978) Design of liposomes for enhanced local release of drugs by hyperthermia. Science 202:1290–1293. https://doi.org/10.1126/science.364652
Nerdal W, Gundersen SA, Thorsen V, Høiland H, Holmsen H (2000) Chlorpromazine interaction with glycerophospholipid liposomes studied by magic angle spinning solid state 13C-NMR and differential scanning calorimetry. Biochim Biophys Acta Biomembr 1464:165–175. https://doi.org/10.1016/S0005-2736(00)00125-5
Alves L, Staneva G, Tessier C, Salgado GF, Nuss P (2011) The interaction of antipsychotic drugs with lipids and subsequent lipid reorganization investigated using biophysical methods. Biochim Biophys Acta Biomembr 1808:2009–2018. https://doi.org/10.1016/j.bbamem.2011.02.021
Evanics F, Prosse RS (2005) Discriminating binding and positioning of amphiphiles to lipid bilayers by 1H NMR. Anal Chim Acta 534:21–29. https://doi.org/10.1016/j.aca.2004.06.061
Avdeef A, Box KJ, Comer JEA, Hibbert C, Tam KY (1998) pH-metric logP 10. Determination of liposomal membrane-water partition coefficients of lonizable drugs. Pharm Res 15:209–215. https://doi.org/10.1023/A:1011954332221
Ståhlberg J (1999) Retention models for ions in chromatography. Chromatogr A 855:3–55. https://doi.org/10.1016/S0021-9673(99)00176-4
Vitovic P, Alakoskela JM, Kinnunen PK (2008) Assessment of drug−lipid complex formation by a high-throughput langmuir-balance and correlation to phospholipidosis. J Med Chem 51:1842–1848. https://doi.org/10.1021/jm7013953
Zhou L, Geraci G, Hess S, Yang L, Wang J, Argikar U (2011) Predicting phospholipidosis: a fluorescence noncell based in vitro assay for the determination of drug-phospholipid complex formation in early drug discovery. Anal Chem 83:6980–6987. https://doi.org/10.1021/ac200683k
Yudate HT, Kai T, Aoki M, Minowa Y, Yamada T, Kimura T, Ono A, Yamada H, Ohno Y, Urushidani T (2012) Identification of a novel set of biomarkers for evaluating phospholipidosis-inducing potential of compounds using rat liver microarray data measured 24-h after single dose administration. Toxicol 295:1–7. https://doi.org/10.1016/j.tox.2012.02.015
Przybylak KR, Cronin MTD (2011) In silico studies of the relationship between chemical structure and drug induced phospholipidosis. Mol Inf 30:415–429. https://doi.org/10.1002/minf.201000164
Ceccarelli M, Wagner B, Alvarez-Sánchez R, Cruciani G, Goracco L (2017) Use of the distribution coefficient in brain polar lipids for the assessment of drug-induced phospholipidosis risk. Chem Res Toxicol 30:1145–1156. https://doi.org/10.1021/acs.chemrestox.6b00459
Takayama N, Lim LW, Takeuchi T (2017) Retention behavior of inorganic anions in hydrophilic interaction chromatography. Anal Sci 33:619–623. https://doi.org/10.2116/analsci.33.619
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwakuma, Y., Okamoto, H., Hamaguchi, R. et al. Immobilized Artificial Membrane Chromatography Using Acetonitrile-Rich Mobile Phase for Comparison of Retention Properties Between Phospholipidosis-Inducing and Non-inducing Basic Drugs. Chromatographia 86, 43–54 (2023). https://doi.org/10.1007/s10337-022-04225-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04225-0