Abstract
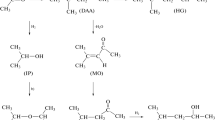
Chromatography is a separation method that utilizes differences in intermolecular interactions of the sample components with the mobile and stationary phases as the sample passes through the column. Acetic acid is a compound of focus in the study of pharmaceutical residues and short-chain fatty acids. Gas chromatography using polar capillary columns (DB-WAX) is an effective means of analyzing acetic acid. In one such solvent, dimethyl sulfoxide (DMSO), the retention time of the acetic acid shows a positive linear correlation with an equal volume increase of the DMSO solvent. We used the quantum chemistry calculation programs ORCA 5.0 and Multiwfn 3.7 with the DFT M062X/6-311 + + G(3d,2p) method to calculate the chromatographic separation parameters. We then analyzed formic acid for comparison and found the retention time of formic acid in the DB-WAX capillary column was longer than that of acetic acid. The retention time variation of formic acid in DMSO solvent compared with aqueous solvent was longer than that of acetic acid; the reason for this observation is that their retention time variation in DMSO solvent was related to the formation of strong hydrogen bonds. There are currently few studies focusing on quantum chemical calculations in chromatographic separations. Our studies using gas chromatography analysis and quantum chemical calculations explain the reasons for retention time variations.






Similar content being viewed by others
References
de Zeeuw J, Luong J (2002) Developments in stationary phase technology for gas chromatography. TrAC, Trends Anal Chem 21(9–10):594–607
Wu Y, Zhang N, Luo K, Liu Y, Bai Z, Tang S. (2022) Recent advances of innovative and high-efficiency stationary phases for chromatographic separations. TrAC Trends in Analytical Chemistry 116647
Zoccali M, Tranchida PQ, Mondello L (2019) Fast gas chromatography-mass spectrometry: a review of the last decade. TrAC, Trends Anal Chem 118:444–452
Špánik I, Machyňáková A (2018) Recent applications of gas chromatography with high-resolution mass spectrometry. J Sep Sci 41(1):163–179
Bartle KD, Myers P (2002) History of gas chromatography. TrAC, Trends Anal Chem 21(9–10):547–557
Makoś P, Przyjazny A, Boczkaj G (2019) Methods of assaying volatile oxygenated organic compounds in effluent samples by gas chromatography—a review. J Chromatogr A 1592:143–160
Ghosh S, AlKafaas SS, Bornman C, Apollon W, Hussien AM, Badawy AE et al (2022) The application of rapid test paper technology for pesticide detection in horticulture crops: a comprehensive review. Beni-Suef Univ J Basic Appl Sci 11(1):73. https://doi.org/10.1186/s43088-022-00248-6
Teglia CM, Montemurro M, De Zan MM, Cámara MS (2015) Multiple responses optimization in the development of a headspace gas chromatography method for the determination of residual solvents in pharmaceuticals. J pharm anal 5(5):296–306
Ren M, Natsagdorj N, Shun N (2022) Influence and mechanism of polar solvents on the retention time of short-chain fatty acids in gas chromatography. Separations 9(5):124
Mu R, Na S, Narantsogt N (2022) Effect of dimethyl sulfoxide on the retention time of acetic acid in gas chromatography and its mechanism. C J Appl Chem 39(5):852–854. https://doi.org/10.1894/j.issn.1000-0518.210175
Panuszko A, Bruździak P, Śmiechowski M, Stasiulewicz M, Stefaniak J, Stangret J (2019) DMSO hydration redefined: unraveling the hydrophobic hydration of solutes with a mixed hydrophilic–hydrophobic characteristic. J Mol Liq 294:111661
Wallace VM, Dhumal NR, Zehentbauer FM, Kim HJ, Kiefer J (2015) Revisiting the aqueous solutions of dimethyl sulfoxide by spectroscopy in the mid-and near-infrared: experiments and Car-Parrinello simulations. J Phys Chem B 119(46):14780–14789
Zhokhov A, Loskutov AY, Rybal’Chenko I (2018) Methodological approaches to the calculation and prediction of retention indices in capillary gas chromatography. J Anal Chem 73(3):207–220
Matyushin DD, Buryak AK (2020) Gas chromatographic retention index prediction using multimodal machine learning. Ieee Access 8:223140–223155
Zhao Y, Truhlar DG (2008) Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J Chem Theory Comput 4(11):1849–1868
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods XX A basis set for correlated wave functions. J Chem Phy 72(1):650–654
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25 supplementary functions for gaussian basis sets. J Chem Phy 80(7):3265–3269
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys rev 136(3B):B864
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):A1133
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies some procedures with reduced errors. Molecular Phy 19(4):553–566
Weinhold F, Glendening ED. NBO 5.0 program manual: natural bond orbital analysis programs.
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102(24):7211–7218. https://doi.org/10.1021/ja00544a007
Świergiel J, Jadżyn J (2018) From supramolecular to conventional polymers: polyethylene glycol. Phys Chem Chem Phys 20(9):6045–6049
Fan X, Su Y, Zhao X, Li Y, Zhang R, Ma T et al (2016) Manipulating the segregation behavior of polyethylene glycol by hydrogen bonding interaction to endow ultrafiltration membranes with enhanced antifouling performance. J Membr Sci 499:56–64
Liu G-K, Zou S, Josell D, Richter LJ, Moffat TP (2018) SEIRAS study of chloride-mediated polyether adsorption on Cu. J Phys Chem C 122(38):21933–21951
Adamska K, Voelkel A (2006) Hansen solubility parameters for polyethylene glycols by inverse gas chromatography. J Chromatogr A 1132(1–2):260–267
de Lima GF, de Souza AG, Rosa DS (2018) Effect of adsorption of polyethylene glycol (PEG), in aqueous media, to improve cellulose nanostructures stability. J Mol Liq 268:415–424
Kou Y, Wang S, Luo J, Sun K, Zhang J, Tan Z et al (2019) Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J Chem Thermodyn 128:259–274
Lai C, Chung C (2020) Hydrophilicity and optic property of polyethylene glycol coating on polydimethylsiloxane for fast prototyping and its application to backlight microfluidic chip. Surf Coat Technol 389:125606
Singh A, Walvekar R, Khalid M, Wong WY, Gupta T (2018) Thermophysical properties of glycerol and polyethylene glycol (PEG 600) based DES. J Mol Liq 252:439–444
Neese F, Wennmohs F, Becker U, Riplinger C (2020) The ORCA quantum chemistry program package. J Chem Phys 152(22):224108
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Ren M, Shun N, Natsagdorj N, Chong-Jiu L (2022) Effect of dimethyl sulfoxide on the retention time of acetic acid in gas chromatography and its mechanism. Chin J Appl Chem 39(5):852
Singh DK, Jagannathan R, Khandelwal P, Abraham PM, Poddar P (2013) In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: an experimental and density functional theory investigation. Nanoscale 5(5):1882–1893
Zhang S, Wang H, Zhu M-J (2019) A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta 196:249–254
Yao L, Davidson EA, Shaikh MW, Forsyth CB, Prenni JE, Broeckling CD (2022) Quantitative analysis of short-chain fatty acids in human plasma and serum by GC–MS. Anal Bioanal Chem 414(15):4391–4399
Ali A, Khalid M, Rehman MFU, Haq S, Ali A, Tahir MN et al (2020) Efficient synthesis SC-XRD and theoretical studies of O-Benzenesulfonylated pyrimidines: role of noncovalent interaction influence in their supramolecular network. ACS Omega 5(25):15115–21528
Peluso P, Mamane V, Dallocchio R, Dessì A, Cossu S (2020) Noncovalent interactions in high-performance liquid chromatography enantioseparations on polysaccharide-based chiral selectors. J Chromatogr A 1623:461202
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7(3):625–632
Contreras-García J, Yang W, Johnson ER (2011) Analysis of hydrogen-bond interaction potentials from the electron density: integration of noncovalent interaction regions. J Phys Chem A 115(45):12983–12990
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Mu Ren, Na Shun, and Sarangerel Davaasambuu wrote the main manuscript text; Ao Rigele, Narantsogt Natsagdorj, and Narmandakh Purev prepared all of the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ren, M., Rigele, A., Davaasambuu, S. et al. Study on Gas Chromatography Retention Time Variation of Acetic Acid Combined with Quantum Chemical Calculation. Chromatographia 86, 3–11 (2023). https://doi.org/10.1007/s10337-022-04220-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04220-5