Abstract
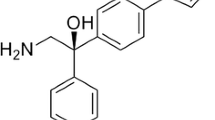
Esaxerenone is a new nonsteroidal mineralocorticoid receptor antagonist utilized to treat high blood pressure. Chemically, esaxerenone is a pyrrole derivative consisting of hindered rotation, which results in stereoisomers named atropisomers. Currently, no methods exist for the separation and quantification of these atropisomers. A new and accurate chiral liquid chromatographic technique was developed and validated to estimate the enantiomeric purity of esaxerenone. Polar organic chiral separation was carried out on an immobilized amylose-based chiral stationary phase (Chiralpak IG) with methanol:acetonitrile:diethylamine (9:1:0.1, v/v/v) mixture as a mobile phase. The total runtime was 15 min, and the resolution (Rs) between the atropisomers was more than 3.0. The detection and quantification thresholds for the R-atropisomer were found to be 0.03 and 0.1 µg mL−1, respectively, for a test concentration of esaxerenone (1000 µg mL−1). Over the range from the limit of quantification to 0.3 percent, the method's linearity for the R-atropisomer was excellent (R2 > 0.999). The R-atropisomer recovery varied from 95 to 102%, confirming the method’s good accuracy. For a 48-h research period, the chemical was shown to be stable.








Similar content being viewed by others
References
Whelton PK (1994) Epidemiology of hypertension. Lancet 344:101–106. https://doi.org/10.1016/s0140-6736(94)91285-8
MacMahon S, Peto R, Collins R et al (1990) Blood pressure, stroke, and coronary heart disease: Part 1, prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 335:765–774. https://doi.org/10.1016/0140-6736(90)90878-9
Funder JW (2013) Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control 6:129–138. https://doi.org/10.2147/IBPC.S13783
Young MJ, Funder JW (2002) Mineralocorticoid receptors and pathophysiological roles for aldosterone in the cardiovascular system. J Hypertens 20:1465–1468. https://doi.org/10.1097/00004872-200208000-00002
Jeunemaitre X, Chatellier G, Kreft-Jais C et al (1987) Efficacy and tolerance of spironolactone in essential hypertension. Am J Cardiol 60:820–825. https://doi.org/10.1016/0002-9149(87)91030-7
Croom KF, Perry CM (2005) Eplerenone: a review of its use in essential hypertension. Am J Cardiovasc Drugs 5:51–69. https://doi.org/10.2165/00129784-200505010-00007
Chapman N, Dobson J, Wilson S et al (2007) Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 49:839–845. https://doi.org/10.1161/01.HYP.0000259805.18468.8c
Sternon J (1990) Spironolactone and altizide in systemic hypertension: ambulatory multicenter study. Am J Cardiol 65:K24–K27. https://doi.org/10.1016/0002-9149(90)91273-9
White WB, Duprez D, St Hillaire R et al (2003) Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension 41:1021–1026. https://doi.org/10.1161/01.HYP.0000067463.13172.EA
The RALES Investigators (1996) Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (The Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 78:902–907. https://doi.org/10.1016/S0002-9149(96)00465-1
Pitt B, Remme W, Zannad F et al (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348:1309–1321. https://doi.org/10.1056/NEJMoa030207
Zannad F, McMurray JJV, Krum H et al (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364:11–21. https://doi.org/10.1056/NEJMoa1009492
Sica DA (2005) Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev 10:23–29. https://doi.org/10.1007/s10741-005-2345-1
Sica DA (2002) Eplerenone: a new aldosterone receptor antagonist—are the fda’s restrictions appropriate. J Clin Hypertens 4:441–445. https://doi.org/10.1111/j.1524-6175.2002.01510.x
Takahashi S, Hiramatsu M, Hotta S et al (2016) Safety and antihypertensive effect of Selara® (Eplerenone): results from a postmarketing surveillance in Japan. Int J Hypertens 2016:e5091951. https://doi.org/10.1155/2016/5091951
Daiichi Sankyo (2019) Daiichi Sankyo announces approval of MINNEBRO(TM) tablets for the treatment of hypertension in Japan - Press Releases - Media - Daiichi Sankyo. Accessed 11 Oct 2021
Ito S, Itoh H, Rakugi H et al (2020) Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension 75:51–58. https://doi.org/10.1161/HYPERTENSIONAHA.119.13569
Arai K, Tsuruoka H, Homma T (2015) CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol 769:266–273. https://doi.org/10.1016/j.ejphar.2015.11.028
Arai K, Morikawa Y, Ubukata N et al (2016) CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther 358:548–557. https://doi.org/10.1124/jpet.116.234765
Kato M, Furuie H, Shimizu T et al (2018) Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics, and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol 84:1821–1829. https://doi.org/10.1111/bcp.13616
Satoh F, Ito S, Itoh H et al (2021) Efficacy and safety of esaxerenone (CS-3150), a newly available nonsteroidal mineralocorticoid receptor blocker, in hypertensive patients with primary aldosteronism. Hypertens Res 44:464–472. https://doi.org/10.1038/s41440-020-00570-5
Yang P, Shen W, Chen X et al (2019) Comparative efficacy and safety of mineralocorticoid receptor antagonists in heart failure: a network meta-analysis of randomized controlled trials. Heart Fail Rev 24:637–646. https://doi.org/10.1007/s10741-019-09790-5
Umemura S (2019) The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res 42:1235–1481. https://doi.org/10.1038/s41440-019-0284-9
PubChem (2021) Esaxerenone, CID: 25052023. https://pubchem.ncbi.nlm.nih.gov/compound/25052023. Accessed 25 Oct 2021
Drugbank (2021) Esaxerenone, Accession number:DB15207. https://go.drugbank.com/drugs/DB15207. Accessed 26 Oct 2021
Yamada M, Takei M, Suzuki E et al (2017) Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective nonsteroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica 47:1090–1103. https://doi.org/10.1080/00498254.2016.1263766
Wipf P, Skoda EM, Mann A (2015) Conformational restriction and steric hindrance in medicinal chemistry. The practice of medicinal chemistry. Elsevier, Amsterdam, pp 279–299
Zask A, Murphy J, Ellestad GA (2013) Biological stereoselectivity of atropisomeric natural products and drugs: biological-stereoselectivity of atropisomeric natural products and drugs. Chirality 25:265–274. https://doi.org/10.1002/chir.22145
LaPlante SR, Fader LD, Fandrick KR et al (2011) Assessing Atropisomer Axial Chirality In Drug Discovery And Development. J Med Chem 54:7005–7022. https://doi.org/10.1021/jm200584g
LaPlante SR, Edwards PJ, Fader LD et al (2011) Revealing atropisomer axial chirality in drug discovery. ChemMedChem 6:505–513. https://doi.org/10.1002/cmdc.201000485
Patel MA, Riley F, Ashraf-Khorassani M, Taylor LT (2012) Supercritical fluid chromatographic resolution of water soluble isomeric carboxyl/amine terminated peptides facilitated via mobile phase water and ion pair formation. J Chromatogr A 1233:85–90. https://doi.org/10.1016/j.chroma.2012.02.024
Yaku K, Aoe K, Nishimura N et al (1997) Chiral resolution of four optical isomers of diltiazem hydrochloride on Chiralcel columns by packed-column supercritical fluid chromatography. J Chromatogr A 785:185–193. https://doi.org/10.1016/S0021-9673(97)00623-7
Loukotková L, Tesařová E, Bosáková Z et al (2010) Comparison of HPLC enantioseparation of substituted binaphthyls on CD-, polysaccharide- and synthetic polymer-based chiral stationary phases: liquid Chromatography. J Sep Science 33:1244–1254. https://doi.org/10.1002/jssc.200900796
Schurig V (2001) Separation of enantiomers by gas chromatography. J Chromatogr A 906:275–299. https://doi.org/10.1016/S0021-9673(00)00505-7
Zerbinati O, Trotta F (2003) pH-dependent cyclodextrin capillary electrophoresis resolution of atropisomers. Electrophoresis 24:2456–2461. https://doi.org/10.1002/elps.200305518
Okamoto Y, Yashima E (1998) Polysaccharide derivatives for chromatographic separation of enantiomers. Angew Chem Int Ed 37:1020–1043. https://doi.org/10.1002/(SICI)1521-3773(19980504)37:8%3c1020::AID-ANIE1020%3e3.0.CO;2-5
Acknowledgements
The authors wish to acknowledge the team of the National Institute of Pharmaceutical Education and Research, Guwahati, and Daicel Chiral Technologies, India, for supporting this work and for their cooperation in performing this work.
Funding
The work was supported by the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Method development and validation were performed by VVSPKR and GS. The first draft of the manuscript was written by VVSPKR, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose. The authors declare that there are no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radhakrishnanand, P., Rayala, V.V.S.P.K., Trivedi, K. et al. Development of Polar Organic Mode Chromatographic Method by Polysaccharide-Based Immobilized Chiral Selector and Validation for the Determination of the Enantiopurity of Novel Mineralocorticoid Receptor Antagonist Atropisomer–Esaxerenone. Chromatographia 85, 553–562 (2022). https://doi.org/10.1007/s10337-022-04164-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04164-w