Abstract
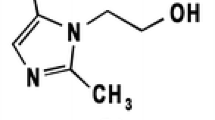
Herein we report two sensitive and accurate UHPLC and TLC-densitometric methods for the simultaneous determination of amprolium HCl, ethopabate, and sulfaquinoxaline-Na. A UHPLC isocratic elution method was adopted with a mobile phase of sodium 1-hexanesulfonate aqueous solution–methanol–acetonitrile in a ratio of (1500:400:100, v/v/v) adjusted to a pH of 5.1 with phosphoric acid. Results showed R2 = 0.9999 over concentration ranges of 0.5–25.0 μg mL−1, 1.0–30.0 μg mL−1, and 1.0–30.0 μg mL−1 for the three drugs, respectively. The accuracy was 100.58% ± 0.52 for amprolium HCl and 98.78% ± 0.54 and 100.14% ± 0.11 for ethopabate and sulfaquinoxaline-Na. Additionally, a TLC-densitometric method was adopted to separate the three cited drugs with a developing system of chloroform:methanol:33% ammonia solution (6:4:0.5 v/v/v) and UV detection at 263 nm. Results showed Rf values of 0.34, 0.65, and 0.95 for amprolium HCl, sulfaquinoxaline-Na, and ethopabate, respectively. The linearity range was 1.0–30.0 μg/band, 0.5–20.0 μg/band, and 1.0–25.0 μg/band for amprolium HCl, ethopabate, and sulfaquinoxaline-Na, respectively. The proposed TLC-densitometric method was utilized for the simultaneous determination of the three drugs in spiked biological matrices with acceptable recoveries. The proposed methods were applied for simultaneous quantitation of three drugs in veterinary formulation and the results were in accordance with those obtained by the reported methods. In conclusion, the two suggested methods were sensitive and accurate for the simultaneous quantitation of the three drugs in both their dosage forms and in biological matrices.




Similar content being viewed by others
References
Girardi C, Odore R (2008) Pharmacological treatments and risks for the food chain. Vet Res Commun 32(Suppl 1):S11–S18. https://doi.org/10.1007/s11259-008-9083-5
Capleton AC, Courage C, Rumsby P et al (2006) Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol Lett 163:213–223. https://doi.org/10.1016/j.toxlet.2005.10.023
Gao F, Yin C-X, Huo F-J, Yang P (2005) Ethopabate. Acta Crystallogr E Struct Rep Online 61:o3870–o3871. https://doi.org/10.1107/S1600536805034082
Clarke L, Fodey TL, Crooks SRH et al (2014) A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci 97:358–374. https://doi.org/10.1016/j.meatsci.2014.01.004
Bedaso K (2016) Comparative study on the efficacy of amprolium and sulfadimidine in coccidia infected chickens in Debre-Zeit agricultural research center poultry farm, Bishoftu, Ethiopia. SOJVS 2:1–5. https://doi.org/10.15226/2381-2907/2/2/00121
Fatoba AJ, Adeleke MA (2018) Diagnosis and control of chicken coccidiosis: a recent update. J Parasit Dis 42:483–493. https://doi.org/10.1007/s12639-018-1048-1
Hussein LA, Magdy N, Abbas MM (2015) Five different spectrophotometric methods for determination of Amprolium hydrochloride and ethopabate binary mixture. Spectrochim Acta A Mol Biomol Spectrosc 138:395–405. https://doi.org/10.1016/j.saa.2014.11.073
Abdelaziz S, Abdel Razeq S, Ahmed N (2021) Smart UV-Spectrophotometric methods for the simultaneous determination of amprolium-HCl, ethopabate and sulfaquinoxaline-Na in combined dosage forms. Azhar Int J Pharmaceutical Med Sci. https://doi.org/10.21608/aijpms.2021.62019.1046
Fink DW, deFontenay G, Bonnefille P et al (2004) Further studies on the spectrophotometric determination of amprolium. J AOAC Int 87:677–680
Catelani TA, Tóth IV, Lima JLFC et al (2014) A simple and rapid screening method for sulfonamides in honey using a flow injection system coupled to a liquid waveguide capillary cell. Talanta 121:281–287. https://doi.org/10.1016/j.talanta.2013.12.034
Alattar A (2018) Native spectrofluorimetric determination of amprolium in bulk and pharmaceutical formulation. Innoriginal: Int J Sci 5(3):42–45
Nasr JJ, Shalan S (2014) Spectrofluorimetric analysis of ethopabate in veterinary formulations with application to residue determination in chicken muscles and liver. Luminescence 29:1188–1193. https://doi.org/10.1002/bio.2683
El-Kosasy AM, Hussein LA, Magdy N, Abbas MM (2015) Sensitive spectrofluorimetric methods for determination of ethopabate and amprolium hydrochloride in chicken plasma and their residues in food samples. Spectrochim Acta A Mol Biomol Spectrosc 150:430–439. https://doi.org/10.1016/j.saa.2015.05.082
Natalia Z, Zholt K, Iryna A et al. (2019) Potentiometric sensor for determination of amprolium in pharmaceutical formulation
Basha MA, Abd El-Rahman MK, Bebawy LI, Salem MY (2017) Novel potentiometric application for the determination of amprolium HCl in its single and combined dosage form and in chicken liver. Chin Chem Lett 28:612–618. https://doi.org/10.1016/j.cclet.2016.11.012
Basha MA, Abd El-Rahman MK, Bebawy LI, Moustafa AA (2019) Validated TLC stability-indicating methods for the quantitative determination of some veterinary drugs. Microchem J 146:157–163. https://doi.org/10.1016/j.microc.2018.12.057
El-Kosasy AM, Lobna AH, Magdy N, Mahmoud MA (2016) Validated TLC–densitometric method for determination of amprolium hydrochloride and ethopabate in veterinary preparation. Anal Chem: Indian J 16(13):1–11
Thomas MH, Soroka KE, Thomas SH (1983) Quantitative thin layer chromatographic multi-sulfonamide screening procedure. J Assoc Off Anal Chem 66:881–883
Goessens T, Baere SD, Troyer ND et al (2020) Highly sensitive multi-residue analysis of veterinary drugs including coccidiostats and anthelmintics in pond water using UHPLC-MS/MS: application to freshwater ponds in Flanders, Belgium. Env Sci Process Impacts 22:2117–2131. https://doi.org/10.1039/d0em00215a
Baker MM, El-Kafrawy DS, Abdel-Khalek MM, Belal TS (2019) Comprehensive stability-indicating high-performance liquid chromatography coupled with diode array detection method for simultaneous determination of amprolium hydrochloride and ethopabate in powder dosage form for veterinary use. J Sep Sci 42:3340–3351. https://doi.org/10.1002/jssc.201900440
Ali MM, Ahmed MAA, Shinger MI (2017) Development and validation of RP-HPLC method for simultaneous determination of amprolium hcl and ethopabate in their combination drug. Chem Biomol Eng 2(1):51–56
Mantri AP, Rubeena MS, Nischal K, Shiva K (2015) Simultaneous estimation of sulfaquinoxaline sodium and amproliumhydrochloride by RP- HPLC. IJPSR 6:1097–1100
Ghanem M, Abu-Lafi S (2013) Validation of a stability-indicating assay of amprolium hydrochloride in water soluble powder formulation using hydrophilic interaction liquid chromatography. J Appl Pharm Sci 3(10):51–58. https://doi.org/10.7324/JAPS.2013.31009
Martínez-Villalba A, Núñez O, Moyano E, Galceran MT (2013) Field amplified sample injection-capillary zone electrophoresis for the analysis of amprolium in eggs. Electrophoresis 34:870–876. https://doi.org/10.1002/elps.201200579
Su HX, Tan HR, Shen HQ, Tian CQ (2013) Simultaneous separation and determination of four anticoccidial drugs in soil by high performance capillary electrophoresis. J Instrum Anal 2:5–10
Van Aken K, Strekowski L, Patiny L (2006) EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J Org Chem 2:3. https://doi.org/10.1186/1860-5397-2-3
Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J (2012) Analytical eco-scale for assessing the greenness of analytical procedures. TrAC, Trends Anal Chem 37:61–72. https://doi.org/10.1016/j.trac.2012.03.013
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Gaber Y, Törnvall U, Kumar MA et al (2011) HPLC-EAT (environmental assessment tool): a tool for profiling safety, health and environmental impacts of liquid chromatography methods. Green Chem 13:2021. https://doi.org/10.1039/c0gc00667j
Mercer SM, Andraos J, Jessop PG (2012) Choosing the greenest synthesis: a multivariate metric green chemistry exercise. J Chem Educ 89:215–220. https://doi.org/10.1021/ed200249v
Tobiszewski M, Marć M, Gałuszka A, Namieśnik J (2015) Green chemistry metrics with special reference to green analytical chemistry. Molecules 20:10928–10946. https://doi.org/10.3390/molecules200610928
Funding
The authors declared no funding was provided for the work published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that for this manuscript, no funds, grants, or other support was received. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Razeq, S.A.A., Aziz, S.E.A. & Ahmed, N.S. TLC–Densitometry and UHPLC Methods for Simultaneous Determination of Amprolium HCl, Ethopabate, and Sulfaquinoxaline-Na in Their New Combined Dosage Form. Chromatographia 85, 563–574 (2022). https://doi.org/10.1007/s10337-022-04163-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04163-x