Abstract
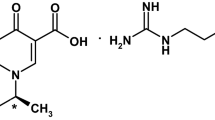
Difluprednate (DFL) is a corticosteroid used topically, especially in the form of emulsion for the treatment of inflammation and pain associated with ocular surgery. Till date, photodegradation of DFL has not been reported in any literature. In this study, a major unknown degradation product was observed after exposure of DFL solution to UV light. Isolation and quantification of this degradation product were performed using RP-HPLC. This major photodegradation product was isolated by using semi-preparative RP-HPLC and its structure was elucidated using mass spectrometry (LC-Q/TOF-HRMS), one and two-dimensional NMR experiments and FT–IR spectroscopy. Based on spectral data, the unknown species was identified as a rearrangement product of the steroid ring, followed by photo-induced elimination of hydrogen fluoride to its final degradant product. The findings of this study can be employed in order to achieve mass balance during photodegradation and to finalize packaging material for storage.






Similar content being viewed by others
References
Zope MV, Patel RM, Patel A, Patel SG (2018) Development and validation of a stability indicating rp-hplc method for the determination of potential degradation products of difluprednate in ophthalmic emulsion. Int J Pharm Pharm Sci 10:79–86. https://doi.org/10.22159/ijpps.2018v10i9.26342
Greaves MW (1976) Anti-inflammatory action of corticosteroids. Postgrad Med J 52(612):631–633. https://doi.org/10.1136/pgmj.52.612.631
Ricci A, Fasani E, Mella M, Albini A (2003) General patterns in the photochemistry of pregna-1, 4-dien-3, 20-diones. J Org Chem 68(11):4361–4366. https://doi.org/10.1021/jo034070a
Albini A, Fasani E, Albini A, Fasani E (1998) Photochemistry of drugs: an overview and practical problems. Drugs Photochem Photostab 225:1–73. https://doi.org/10.1039/9781847550712-00001
Miolo G, Gallocchio F, Levorato L, Dalzoppo D, van Henegouwen GMB, Caffieri S (2009) UVB photolysis of betamethasone and its esters: characterization of photoproducts in solution, in pig skin and in drug formulations. J Photochem Photobiol B Biol 96(1):75–81. https://doi.org/10.1016/j.jphotobiol.2009.04.007
Lin M, Li M, Buevich AV, Osterman R, Rustum AM (2009) Rapid structure elucidation of drug degradation products using mechanism-based stress studies in conjunction with LC–MSn and NMR spectroscopy: identification of a photodegradation product of betamethasone dipropionate. J Pharm Biomed Anal 50(3):275–280. https://doi.org/10.1016/j.jpba.2009.04.004
Bhutnar A, Khapare S, Desai A, Dsouza S (2017) Isolation and characterization of photodegradation impurity in budesonide drug product using LC-MS and NMR spectroscopy. Am J Analyt Chem 8(7):449–461. https://doi.org/10.4236/ajac.2017.87034
Ahmad I, Ahmed S, Anwar Z, Sheraz MA, Sikorski M (2016) Photostability and photostabilization of drugs and drug products. Int J Photoenergy. https://doi.org/10.1155/2016/8135608
Sambandan E, Kathavarayan T, Sellappan S, Shiea J, Ponnusamy VK (2019) Identification and characterization of unknown degradation impurities in beclomethasone dipropionate cream formulation using HPLC, ESI-MS and NMR. J Pharm Biomed Anal 167:123–131. https://doi.org/10.1016/j.jpba.2019.02.013
ICH tripartite guideline (1996) STABILITY TESTING: PHOTOSTABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS Q1B. https://database.ich.org/sites/default/files/Q1B%20Guideline.pdf Accessed 14 Mar 2022
ICH tripartite guideline (2006) Impurities in new drug substances Q3A(R2) https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf Accessed 14 Mar 2022
ICH tripartite guideline (2006) Impurities in new drug products Q3B(R2) https://database.ich.org/sites/default/files/Q3B%28R2%29%20Guideline.pdf Accessed 14 Mar 2022
Banerjee P, Eckert AO, Schrey AK, Preissner R (2018) ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 46(W1):W257–W263. https://doi.org/10.1093/nar/gky318
Chavan BB, Sawant V, Borkar RM, Ragampeta S, Talluri MK (2019) Isolation and structural characterization of degradation products of afatinib dimaleate by LC-Q-TOF/MS/MS and NMR: cytotoxicity evaluation of afatinib and isolated degradation products. J Pharm Biomed Anal 166:139–146. https://doi.org/10.1016/j.jpba.2019.01.004
Gu ZM, Ma J, Zhao XG, Wu J, Zhang D (2006) Reduction of nitriles to amines in positive ion electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 20(19):2969–2972. https://doi.org/10.1002/rcm.2690
Williams JR, Moore RH, Li R, Weeks CM (1980) Photochemistry of 11. alpha-and 11. Beta.-hydroxy steroidal 1, 4-dien-3-ones and 11. alpha-and 11. beta-.hydroxy steroidal bicyclo [3.1. 0] hex-3-en-2-ones in neutral and acidic media. J Org Chem 45(12):2324–2331. https://doi.org/10.1021/jo01300a012
Ogata M, Noro Y, Yamada M, Tahara T, Nishimura T (1998) Photodegradation products of methylprednisolone suleptanate in aqueous solution—evidence of a bicyclo [3.1. 0] hex-3-en-2-one intermediate. J Pharm Sci 87(1):91–95. https://doi.org/10.1021/js9701115
Shirasaki Y, Inada K, Inoue J, Nakamura M (2004) Isolation and structure elucidation of the major photodegradation products of loteprednol etabonate. Steroids 69(1):23–34. https://doi.org/10.1016/j.steroids.2003.09.010
Hidaka T, Huruumi S, Tamaki S, Shiraishi M, Minato H (1980) Studies on betamethasone: behavior of betamethasone in acid or alkaline medium, photolysis, and oxidation. Yakugaku Zasshi 100(1):72–80. https://doi.org/10.1248/yakushi1947.100.1_72
Acknowledgements
We would like to thank National Institute of Pharmaceutical Education and Research, Ahmedabad for providing us with required support, infrastructure and access to literature throughout the course of this work.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, N., Giri, S., Sahu, A.k. et al. LC-Q/TOF-HRMS and NMR Based Structural Characterization of the Major Photodegradation Impurity of Difluprednate. Chromatographia 85, 605–615 (2022). https://doi.org/10.1007/s10337-022-04159-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04159-7