Abstract
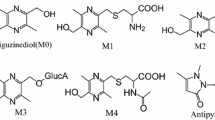
Daphnetin, which has been developed as a drug against obliterative vasculitis, can be rapidly and stereoselectively metabolized to an active 8-O-methylated metabolite, namely daphnetin 8-methyl ether (daphnetin-Me). Herein, a rapid, sensitive and reliable ultrafast liquid chromatography tandem mass spectrometry (UFLC-MS/MS) method was developed and validated to simultaneously determine daphnetin and daphnetin-Me in rat plasma after intragastric administration. The MS quantification for the two analytes and 3-aminocoumarin (internal standard, IS) was carried out on a triple quadrupole mass spectrometer using an ESI source in positive multiple reaction monitoring mode (daphnetin: m/z 179.15 → 51.10; daphnetin-Me: m/z 193.30 → 150.05; IS: m/z 162.00 → 106.20). The method exhibited a broad linear range of 1–2000 ng mL−1. The intra- and inter- assay precisions (RSD%) were ≤ 8.29% with the accuracies (RME%) within ± 5.95%. This newly developed method was successfully applied to a pharmacokinetic study of daphnetin after a single dose of 20 mg kg−1 in rats. Daphnetin and daphnetin-Me peaked almost at the same time. Compared with that of daphnetin, a 2.1-fold higher area under the concentration–time curve (AUC) for daphnetin-Me were observed. These results would be beneficial in facilitating further investigation of pharmacological mechanisms, as well as the rational application of daphnetin and daphnetin-containing herb preparations.




Similar content being viewed by others
Abbreviations
- COMT:
-
Catechol-O-methyltransferase
- IS:
-
Internal standard
- ESI:
-
Electrospray ionization
- UFLC:
-
Ultra-fast liquid chromatography
- NMR:
-
Nuclear magnetic resonance
- LLOQ:
-
Lower limits of quantification
- MRM:
-
Multiple reaction monitoring
- PK:
-
Pharmacokinetic
- C max :
-
Maximum concentration
- T max :
-
Time to Cmax
- T 1/2 :
-
Terminal elimination half-life
- AUC:
-
Area under curve
- MRT:
-
Mean residence times
References
Obach RS (2013) Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev 65:578–640
Park BK, Boobis A, Clarke S et al (2011) Managing the challenge of chemically reactive metabolites in drug development. Nat Rev Drug Discov 10:292–306
Ezzeldin E, Abo-Talib NF, Tammam MH (2017) UPLC-tandem mass spectrometry method for simultaneous determination of fluoxetine, risperidone, and its active metabolite 9-hydroxyrisperidone in plasma: application to pharmacokinetics study in rats. J Anal Methods Chem 2017:5187084
Food and Drug Administration (FDA) (2020) Guidance for industry: safety testing of drug metabolites. MD, USA
European Medicines Agency (2009) ICH guideline M3(R2) on non-clinical safety studies for the conduct of human clinical trials and marketing authorisation for pharmaceuticals, London, UK.
Mazak K, Noszal B, Hosztafi S (2017) Physicochemical and pharmacological characterization of permanently charged opioids. Curr Med Chem 24:3633–3648
Del RD, Rodriguez-Mateos A, Spencer JP et al (2013) Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 18:1818–1892
Müller T (2010) Entacapone. Expert Opin Drug Metab Toxicol 6:983–993
LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord 30:64–72
Liang SC, Xia YL, Hou J et al (2016) Methylation, glucuronidation, and sulfonation of daphnetin in human hepatic preparations in vitro: metabolic profiling, pathway comparison, and bioactivity analysis. J Pharm Sci 105:808–816
Cavalieri EL, Rogan EG, Chakravarti D (2002) Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell Mol Life Sci 59:665–681
Antonio L, Grillasca JP, Taskinen J et al (2002) Characterization of catechol glucuronidation in rat liver. Drug Metab Dispos 30:199–207
Antonio L, Xu J, Little JM et al (2003) Glucuronidation of catechols by human hepatic, gastric, and intestinal microsomal UDP-glucuronosyltransferases (UGT) and recombinant UGT1A6, UGT1A9, and UGT2B7. Arch Biochem Biophys 411:251–261
Cavalieri EL, Li KM, Balu N et al (2002) Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis 23:1071–1077
Okoko T, Oruambo IF (2009) Inhibitory activity of quercetin and its metabolite on lipopolysaccharide-induced activation of macrophage U937 cells. Food Chem Toxicol 47:809–812
Xu H, Li Y, Che X et al (2014) Metabolism of salvianolic acid A and antioxidant activities of its methylated metabolites. Drug Metab Dispos 42:274–281
Poór M, Zrínyi Z, Kőszegi T et al (2016) Structure related effects of flavonoid aglycones on cell cycle progression of HepG2 cells: metabolic activation of fisetin and quercetin by catechol-O-methyltransferase (COMT). Biomed Pharmacother 83:998–1005
Han S, Li LZ, Song SJ et al (2020) Daphne giraldii Nitsche (Thymelaeaceae): phytochemistry, pharmacology and medicinal uses. Phytochemistry 171:112231
Yeşilada E, Taninaka H, Takaishi Y et al (2001) In vitro inhibitory effects of Daphne oleoides ssp. oleoides on inflammatory cytokines and activity-guided isolation of active constituents. Cytokine 13:359–364
Ji J, Ge X, Chen Y et al (2019) Daphnetin ameliorates experimental colitis by modulating microbiota composition and T(reg)/T(h)17 balance. FASEB J 33:9308–9322
Liang SC, Ge GB, Liu HX et al (2010) Identification and characterization of human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of daphnetin. Drug Metab Dispos 38:973–980
Liang SC, Ge GB, Xia YL et al (2015) In vitro evaluation of the effect of 7-methyl substitution on glucuronidation of daphnetin: metabolic stability, isoform selectivity, and bioactivity analysis. J Pharm Sci 104:3557–3564
Liang SC, Ge GB, Xia YL et al (2017) In vitro metabolism of daphnetin in rat liver S9 fractions. Yao Xue Xue Bo 52:291–295
Walle T, Wen X, Walle UK (2007) Improving metabolic stability of cancer chemoprotective polyphenols. Expert Opin Drug Metab Toxicol 3:379–388
Ma Z, Liu H, Wu B et al (2014) Structure-based drug design of catechol-O-methyltransferase inhibitors for CNS disorders. Br J Clin Pharmacol 77:410–420
Zhang W, Di LQ, Li JS et al (2014) The effects of Glycyrrhizae uralenis and its major bioactive components on pharmacokinetics of daphnetin in Cortex daphnes in rats. J Ethnopharmacol 154:584–592
Shan J, Qian W, Peng L et al (2018) A comparative pharmacokinetic study by UHPLC-MS/MS of main active compounds after oral administration of Zushima-Gancao extract in normal and adjuvant-induced arthritis rats. Molecules 23:227
Han D, Chen C, Zhang C, Zhang Y, Tang X (2010) Determination of mangiferin in rat plasma by liquid-liquid extraction with UPLC-MS/MS. J Pharm Biomed Anal 51:260–263
Food and Drug Administration (2018) Guidance for Industry: bioanalytical method validation. US Department of Health and Human Services, US. FDA.
De Nicolò A, Cantù M, D’Avolio A (2017) Matrix effect management in liquid chromatography mass spectrometry: the internal standard normalized matrix effect. Bioanalysis 9:1093–1105
Song M, Yu X, Zhao H et al (2009) LC-MS-MS determination and pharmacokinetic study of clozapine in human plasma. Chromatographia 69:1049–1054
Sun C, Wang Y, Sun S et al (2020) Fragmentation pathways of protonated coumarin by ESI-QE-orbitrap-MS/MS coupled with DFT calculations. J Mass Spectrom 55:e4496
Yuan W, Wang J, An X (2021) UPLC-MS/MS method for the determination of hyperoside and application to pharmacokinetics study in rat after different administration routes. Chromatographia 84:249–256
Qu SY, Wu YJ, Wang YH et al (1983) Metabolism and pharmacokinetics of daphnetin. Yao Xue Xue Bo 18:496–500
Hen L, Di L, Liu H et al (2011) Effects of glycyrrhiza extract on pharmacokinetics property of daphnetin in rats. Chin J Tradit Chin Med 36:935–938
Du Q, Di LQ, Chan JJ et al (2009) Intestinal absorption of daphnetin by rats single pass perfusion in situ. Yao Xue Xue Bo 8:922–926
Acknowledgements
This work was supported by the National Natural Science Foundation (81672458, 82003850), the Grants from the Science and Technology Planning Project of Sichuan Province (2019JDPT0010, 2021JDTD0003), the China Postdoctoral Science Foundation (2020M673290), the Sichuan Province Post Doctoral Fund Special Assistance Program (202027), and the Joint Fund of Luzhou City and Southwest Medical University (2018LZXNYD-ZK18, 2018LZXNYD-ZK28). The authors gratefully acknowledge Prof. Jianming Wu for valuable suggestions, and Mr. Linjie Zhu for research assistance. We thank Dr. Jiangwei Zhang for improving the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, C., He, B. et al. Determination of Daphnetin and its 8-O-Methylated Metabolite in Rat Plasma by UFLC-MS/MS: Application to a Pharmacokinetic Study. Chromatographia 85, 333–341 (2022). https://doi.org/10.1007/s10337-022-04131-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04131-5