Abstract
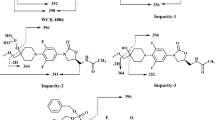
WCK 771 is a novel antibacterial drug recently launched in India for the treatment of acute bacterial skin and skin structure infections (ABSSSI). This report describes identification, quantification of impurities and degradation products (DPs) in the drug substance. The research embraces development of two reversed phase liquid chromatographic (RP-LC) methods; one mass spectrometer (MS) compatible method for identification and another UV detection method for the quantification of impurities. Four process impurities and eight DPs were identified using liquid chromatography tandem mass spectrometry (LC–MS/MS). The chromatographic separation was achieved on C18 column applying gradient elution. WCK 771 was subjected to hydrolytic, oxidative, photolytic and thermal stress. Degradation was observed only in acidic and oxidative environment. Validated UV method is accurate and precise to quantify impurities with accuracy greater than 98.71% and precision of less than 2.82%. The method is highly sensitive to detect impurities as low as 0.17 ng on column. The developed method is employed for quality control and stability studies of the new drug substance.






Similar content being viewed by others
References
De Souza NJ, Deshpande PK, Shukla MC, Mukaram SMJ, Kulkarni DG, Ansari AR, Yeole RD, Patel MV, Gupte SV (2003) Crystalline fluoroquinolone arginine salt form. Google Patents (US Patent No, US6664267B1)
De Souza NJ, Gupte SV, Deshpande PK, Desai VN, Bhavsar SS, Yeole RD (2005) A chiral benzoquinolizine-2-carboxylic acid arginine salt active against vancomycin-resistant Staphylococcus aureus. J Med Chem 48:5232–5242. https://doi.org/10.1021/jm050035f
Appelbaum PC, Pankuch GA, Bozdogan B, Lin G, Jacobs MR, Patel MV, Gupte SV, Jafri MA, De Sourza NJ, Khorakiwala HF (2004) Activity of the new quinolone WCK 771 against pneumococci. Clin Micro Infect Dis 11:9–14. https://doi.org/10.1111/j.1469-0691.2004.01017.x
Flamm RK, Farrell DJ, Sader HS, Rhomberg PR, Jones RN (2016) In vitro activity of WCK 771, a benzoquinolizine fluoroquinolone (Levonadifloxacin) when tested against contemporary Gram-positive and negative bacteria from a global surveillance program. Abstract Sunday-456. ASM Microbe, Boston, MA. American Society for Microbiology, Washington
Bhagwat S, Nandanwar M, Kansagara A, Patel A, Takalkar S, Chavan R, Periasamy H, Yeole R, Deshapane PK, Bhavsar S, Bhatia A, Ahdal J, Jain R, Patel M (2019) Levonadifloxacin, a novel broad-spectrum anti-MRSA benzoquinolizine quinolone agent: review of current evidence. Drug Des Dev Ther 13:1351–1365. https://doi.org/10.2147/DDDT.S229882
Saxena D, Kaul G, Dasgupta A, Chopra S (2020) Levonadifloxacin arginine salt to treat acute bacterial skin and skin structure infection due to S. aureus including MARSA. Drug Today 56(9):583–598. https://doi.org/10.1358/dot.2020.56.9.3168445
ICH guideline (2006) Q3 (R2) Impurities in new drug substances, International Conference on Harmonization, IFPMA, Geneva
ICH guideline (1999) Q6A Specifications: Test procedures and acceptance criteria for new drug substances and new drug products: Chemical substances, International Conference on Harmonization, IFPMA, Geneva
Yeole RD, Jadhav AS, Patil KR, Rane VP, Kubal ML, Singh S, Patel MV, Khorakiwala HF (2006) Validated chiral high-performance liquid chromatography method for a novel anti-methicillin-resistant Staphylococcus aureus fluoroquinolone WCK 771. J Chromatogr A 1108:38–42. https://doi.org/10.1016/j.chroma.2005.12.085
Yeole RD, Kulkarni VL, Latad S, Chavan RP, Chugh Y, Patel MV, Khorakiwala HF (2007) Simple liquid chromatography-tandem mass spectrometry method for determination of novel anti-methicillin-resistant Staphylococcus aureus fluoroquinolone WCK 771 in human serum. J Chromatogr B 846:306–312. https://doi.org/10.1016/j.jchromb.2006.09.022
Yeole RD, Rane VP, Ahirrao VK, Chavan RP, Patel AM, Deshpande PK, Patel MV, Patil KR (2019) Identification of metabolites of novel anti-MRSA fluoroquinolone WCK 771 in mice, rat, rabbit, dog, monkey and human urine using liquid chromatography tandem mass spectrometry. Biomed Chromatog 33:e4532. https://doi.org/10.1002/bmc.4532
Patil KR, Rane VP, Ahirrao VK, Yeole RD (2020) Impurity profiling of a novel anti-MRSA antibacterial drug: alalevonadifloxacin. J Chromatog Sci 58:951–960. https://doi.org/10.1093/chromsci/bmaa068
Devhadrao N, Bansode A, Yeole R (2014) Analytical method development and validation of S-nadifloxacin in pure form by HPLC. Int J Pharm Clin Res 6:63–67
Kulkarni AA, Nanda RK, Ranjane MN, Ranjane PN (2010) Simultaneous estimation of S-nadifloxacin and mometasone furoate in topical cream by HPTLC method. Der Pharma Chemica 2:25–30
Kumar A, Sinha S, Agrawal S, Ali J, Ahuja A, Baboota S (2010) Validated stability indicating thin layer chromatographic determination of S-nadifloxacin in microemulsion and bulk drug formulations. J Food Drug Anal 18:358–365. https://doi.org/10.38212/2224-6614.2276
Bhosale DM, Nikalge AP (2017) Stability indicating UPLC method for the estimation of nadifloxacin, terbinafine hydrochloride, mometasone furoate, methyl paraben and propyl paraben in topical pharmaceutical dosage form. Drug Form Clin Methods 100:1407–1413. https://doi.org/10.5740/jaoacint.16-0254
Salunke N, Pandita N (2018) Stability indicating LC-DAD method for determination of nadifloxacin and characterization of its degradation products by LC-ESI-MS/MS. Chromatographia 81:469–478. https://doi.org/10.1007/s10337-018-3468-6
Kilbile JT, Tamboli Y, Rafeeq M, Yadav RP, Rane VP, Bhamare VS, Merwade AY (2021) Efficient synthesis of potential impurities in Levonadifloxacin (WCK 771). ACS Omega. https://doi.org/10.1021/acsomega.1c04639
Salem MY, El Guindi NM, Mikael HK, Fattah LS (2006) Stability indicating methods for the determination of some fluoroquinolones in the presence of their decarboxylated degradants. Chem Pharma Bull 54:1625–1632. https://doi.org/10.1248/cpb.54.1625
Zheng YJ, He JM, Zhang RP, Wang YC, Wang JX, Wang HQ, Wu Y, He WY, Abliz Z (2014) An integrated approach for detection and characterization of the trace impurities in levofloxacin using liquid chromatography—tandem mass spectrometry. Rapid Comm Mass Spectrom 28:1164–1174. https://doi.org/10.1002/rcm.6886
Salunke N, Kharkar PS, Pandita N (2019) Study of degradation behavior of besifloxacin, characterization of its degradation products by LC-ESI-QTOF-MS and their silico toxicity prediction. Biomed Chromatog. https://doi.org/10.1002/bmc.4489
Barbara ZW, Pawel Z, Marek A, Anna M, Hubicka U (2020) Development and validation of stability indicating HPLC methods for the estimation of Lomefloxacin and Balofloxacin oxidation process under ACVA, H2O2, KMNO4 treatment, kinetic evaluation and identification of degradation products by mass spectrometry. Molecules 25:5251. https://doi.org/10.3390/molecules25225251
Liu ZY, Zhou XN, Zhang HH, Warn L, Sun ZL (2011) An integrated method for degradation products detection and characterization using hybrid ion trap/time of flight mass spectrometry and data processing techniques: applications to study of the degradation products of danofloxacin under stressed conditions. Anal Bioanal Chem 399:2475–2486. https://doi.org/10.1007/s00216-010-4629-0
ICH guideline (2005) Q2 (R1)—validation of analytical procedures: text and methodology, International Conference on Harmonization, IFPMA, Geneva
Acknowledgements
The authors acknowledge the discussions with Dr. Vijay Chavan and Dr. Yasinalli Tamboli in predicting the structures of degradation products.
Funding
The research work presented in this manuscript is a part of drug discovery research program of Wockhardt Limited.
Author information
Authors and Affiliations
Contributions
VPR: UV based LC method development and drafting the manuscript. RP: conducted forced degradation studies. KP: MS compatible LC method development. AP: LC–MS analysis. SN: LC–MS/MS analysis. VKA: Review of method validation data and editing of manuscript. RJ: Application of LC method for analysis of batches. RDY: Project administration, planning of experiments, interpretation of data, review and editing of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The presented work is original research work of Wockhardt Limited and all the authors are employees of Wockhardt Limited.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rane, V.P., Patil, R.H., Patil, K.R. et al. Investigation on Impurity Profiling and Degradation Behavior of WCK 771. Chromatographia 85, 155–165 (2022). https://doi.org/10.1007/s10337-021-04122-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04122-y