Abstract
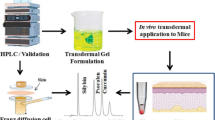
A novel HPLC method was developed and validated to simultaneously determine curcuminoids and dasatinib in a new dosage form. Excellent separation was achieved for a curcuminoids mixture and dasatinib with a ZORBAX Eclipse Plus Phenyl–Hexyl column (4.6 × 250 mm, 5 µm) and a mobile phase consisting of aqueous solution (0.2% acetic acid and 0.1% trifluoroacetic acid with the pH adjusted to 3.5) and acetonitrile with a gradient elution at a flow rate of 1 mL/min. The separated peaks were detected at 325 nm for dasatinib and 420 nm for the curcuminoids mixture. The mixture was separated in less than 20 min. The retention times were 6.5 min, 16.4 min, 17.5 min, and 18.4 min for dasatinib, bisdesmethoxycurcumin, desmethoxycurcumin, and curcumin, respectively. The linear range for all compounds was 0.5–140 µg/mL with a limit of detection of 0.10–0.50 µg/mL. The method was validated according to the International Council for Harmonization guidelines and was shown to be sensitive, fast, robust, specific, and accurate. The developed method was successfully applied to determine dasatinib and curcuminoids in a nanoparticle preparation with good accuracy and precision. The method may be utilized to conduct in vitro studies for new pharmaceutical formulations containing a similar combination of compounds.














Similar content being viewed by others
References
Sun W, Sanderson PE, Zheng W (2016) Drug combination therapy increases successful drug repositioning. Drug Discov Today 21(7):1189–1195
FDA, U. Analytical procedures and methods validation for drugs and biologics guidance for industry. 2015; 18]. Available from: https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf.
Coskun O (2016) Separation techniques: chromatography. North Clin Istanb 3(2):156–160
Selvam C et al (2019) Molecular mechanisms of curcumin and its analogs in colon cancer prevention and treatment. Life Sci 239:117032
Kotra VSR, Satyabanta L, Goswami TK (2019) A critical review of analytical methods for determination of curcuminoids in turmeric. J Food Sci Technol 56(12):5153–5166
Rodrigues FC, Anil Kumar NV, Thakur G (2019) Developments in the anticancer activity of structurally modified curcumin: an up-to-date review. Eur J Med Chem 177:76–104
Perkins S et al (2002) Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomark Prev 11(6):535–540
Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15(1):195–218
Shehzad A, Wahid F, Lee YS (2010) Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm 343(9):489–499
Hatcher H et al (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65(11):1631–1652
Naksuriya O et al (2014) Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 35(10):3365–3383
Sharma RA et al (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10(20):6847–6854. https://doi.org/10.1158/1078-0432.CCR-04-0744
Araujo J, Logothetis C (2010) Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev 36(6):492–500
Kim LC, Rix U, Haura EB (2010) Dasatinib in solid tumors. Expert Opin Investig Drugs 19(3):415–425
Serrels A et al (2006) Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther 5(12):3014–3022
Nautiyal J et al (2009) Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett 283(2):143–151
Wang B-L et al (2013) Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int J Nanomed 8:3521
Hosseinzadeh L et al (2011) Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food Chem Toxicol 49(5):1102–1109
Huang Y-F et al (2017) Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget 8(25):40264
Chen P et al (2015) Curcumin reverses cisplatin resistance in cisplatin-resistant lung caner cells by inhibiting FA/BRCA pathway. Tumor Biology 36(5):3591–3599
Zhang J et al (2017) Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother Pharmacol 79(3):479–487
Zhao M-D et al (2019) Co-delivery of curcumin and paclitaxel by “core-shell” targeting amphiphilic copolymer to reverse resistance in the treatment of ovarian cancer. Int J Nanomed 14:9453
Tian N, Shangguan W, Zhou Z, yao Y, Fan C, Cai L, (2019) Lin28b is involved in curcumin-reversed paclitaxel chemoresistance and associated with poor prognosis in hepatocellular carcinoma. J Cancer 10(24):6074–6087
Abd Wahab NA et al (2020) Mechanism of anti-cancer activity of curcumin on androgen-dependent and androgen-independent prostate cancer. Nutrients 12(3):679
Ismail NI et al (2019) Mechanism of apoptosis induced by curcumin in colorectal cancer. Int J Mol Sci 20(10):2454
He YC et al (2019) Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules 24(22):4179
Sun A et al (2009) Curcumin analog cytotoxicity against breast cancer cells: exploitation of a redox-dependent mechanism. Bioorg Med Chem Lett 19(23):6627–6631
Carolina Alves R et al (2019) A critical review of the properties and analytical methods for the determination of curcumin in biological and pharmaceutical matrices. Crit Rev Anal Chem 49(2):138–149
Gonzalez AG et al (2017) Determination of dasatinib in the tablet dosage form by ultra high performance liquid chromatography, capillary zone electrophoresis, and sequential injection analysis. J Sep Sci 40(2):400–406
Agarwal S et al (2019) Development of Chromatographic Method for Determination of Impurities in Solid Dispersion of Dasatinib. Brazi J Anal Chem 5(21):19–29
Gugulothu DB, Patravale VB (2012) A new stability-indicating hplc method for simultaneous determination of curcumin and celecoxib at single wavelength: an application to nanoparticulate formulation. Pharm Anal Acta 03(04):157
Horská J et al (2014) CZE separation of new drugs for treatment of leukemia. Chromatographia 77(21–22):1477–1482
Korany MA et al (2017) A validated stability-indicating HPLC method for simultaneous determination of Silymarin and Curcumin in various dosage forms. Arab J Chem 10:S1711–S1725
Vieira E, Lemos-Senna E (2020) Application of a new validated HPLC-PDA method for simultaneous determination of curcumin and melatonin in hyaluronic acid-coated nanoemulsions. J Brazil Chem Soc 31(3):467–475
Ruan L et al (2015) A sensitive and microscale method for drug screening combining affinity probes and single molecule fluorescence correlation spectroscopy. Analyst 140(4):1207–1214
Jesus CSH, Diculescu VC (2015) Redox mechanism, spectrophotometrical characterisation and voltammetric determination in serum samples of kinases inhibitor and anticancer drug dasatinib. J Electroanal Chem 752:47–53
Kalambate PK et al (2019) Mesoporous Pd@Pt core–shell nanoparticles supported on multi-walled carbon nanotubes as a sensing platform: application in simultaneous electrochemical detection of anticancer drugs doxorubicin and dasatinib. Anal Methods 11(4):443–453
Xu M et al (2018) Screening of break point cluster region Abelson tyrosine kinase inhibitors by capillary electrophoresis. J Chromatogr A 1537:128–134
Kharat S, Namdeo A, Mehta P (2018) Development and validation of HPTLC method for simultaneous estimation of curcumin and galangin in polyherbal capsule dosage form. J Taibah Uni Sci 11(5):775–781
Mhaske DV, Dhaneshwar SR (2007) Stability indicating HPTLC and LC determination of dasatinib in pharmaceutical dosage form. Chromatographia 66(1–2):95–102
Peram MR et al (2017) Stability studies of pure and mixture form of curcuminoids by reverse phase-HPLC method under various experimental stress conditions. Food Sci Biotechnol 26(3):591–602
Xu H et al (2018) Multiplexed quantitative MALDI MS approach for assessing activity and inhibition of protein kinases based on postenrichment dephosphorylation of phosphopeptides by metal-organic framework-templated porous CeO2. Anal Chem 90(16):9859–9867
Yi R et al (2019) Identification of ligands from natural products as inhibitors of glutathione S-transferases using enzyme immobilized mesoporous magnetic beads with high-performance liquid chromatography plus quadrupole time-of-flight mass spectrometry and molecular docking. J Sep Sci 42(24):3611–3620
Sun QX et al (2019) Single cell analysis for elucidating cellular uptake and transport of cobalt curcumin complex with detection by time-resolved ICPMS. Anal Chim Acta 1066:13–20
Sahu A et al (2011) Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J Biomater Appl 25(6):619–639
Akbar MU et al (2018) Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS PharmSciTech 19(6):2719–2739
Roy S, Quinones R, Matzger AJ (2012) Structural and physicochemical aspects of dasatinib hydrate and anhydrate phases. Cryst Growth Des 12(4):2122–2126
Poole, C.F. and N. Lenca, Chapter 4—Reversed-phase liquid chromatography, in Liquid Chromatography (Second Edition), S. Fanali, et al., Editors. 2017, Elsevier. p. 91–123.
Acknowledgements
The authors would like to acknowledge the financial support provided by King Abdulaziz City for Science and Technology (KACST), Grant No. 2-17-03-007-0010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alarjah, M.A., Shahin, M.H., Al-Azzah, F. et al. Concomitant analysis of dasatinib and curcuminoids in a pluronic-based nanoparticle formulation using a novel HPLC method. Chromatographia 83, 1355–1370 (2020). https://doi.org/10.1007/s10337-020-03956-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03956-2