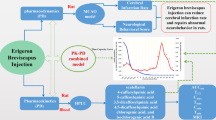
Abstract
To explore the pharmacokinetic characteristics of the three monoterpene glycosides (paeoniflorin, albiflorin and oxypaeoniflorin) extracted from the Radix Paeoniae Rubra (RPR), we determined the plasma concentrations of these components in the cerebral ischemia–reperfusion (CIR) rats and the normal rats by a self-developed and validated ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS–MS) method. In this study, the CIR rats and the normal rats both underwent an intravenous administration of total paeony glucosides (TPG). The blood samples were collected from rats by eye puncture at a series of time points. Then, the pharmacokinetic parameters of the three components were calculated, including the half-life (t1/2), the area under curve of the concentration–time (AUC), the mean residence time (MRT), apparent volume of distribution (Vz) and the clearance (CL). Studies demonstrated that the Vz of albiflorin, the Vz of oxypaeoniflorin, the t1/2 of oxypaeoniflorin and the MRT of oxypaeoniflorin increased in the CIR rats versus the normal rats. Moreover, we found albiflorin and oxypaeoniflorin showed smaller AUC, longer MRT, larger Vz and larger CL than paeoniflorin both in CIR rats and normal rats. In conclusion, we used a validated method to determine the pharmacokinetic characteristics of three monoterpene glycosides in both CIR rats and normal rats simultaneously. These results demonstrated that there were meaningful differences among the pharmacokinetics of the three monoterpene glycosides in TPG either in normal rats or CIR rats. And the CIR model had influences on the in vivo process both of albiflorin and oxypaeoniflorin.



Similar content being viewed by others
Code Availability
All software used in this research were declared in the experimental section in the manuscript.
Availability of Data and Material
Data that support this research were represented in Table attached to the manuscript.
References
Powers WJ (1991) Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29(3):231–240
Ignarro LJ, Balestrieri ML, Napoli C (2007) Nutrition, physical activity, and cardiovascular disease: an update. Cardiovasc Res 73(2):326–340
Furie K et al (2010) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:227–276
Knopman DS, Hooshmand B (2017) Cerebrovascular disease affects brain structural integrity long before clinically overt strokes. Neurology 89(2):1–2
Hu X et al (2017) Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 120(3):449–471
Donnan GA et al (2008) Stroke. Lancet 371(9624):1612–1623
Jin Y et al (2013) Buyang Huanwu Decoction fraction protects against cerebral ischemia/reperfusion injury by attenuating the inflammatory response and cellular apoptosis. Neural Regen Res 8(3):197–207
Ren-Qiang MA et al (2005) Protective effects of total paeony glycoside against global cerebral ischemia-reperfusion injury in gerbils. J First Mil Med Univ 25(4):471–473
He YL, Zhou D (2006) Protective effects of total paeony glycoside against focal cerebral ischemia/reperfusion injury in rats. Chin J Clin Rehabilit 10(39):59–61
He X et al (2004) Effects of cerebral ischemia-reperfusion on pharmacokinetic fate of paeoniflorin after intravenous administration of Paeoniae Radix extract in rats. J Ethnopharmacol 94(2):339–344
Guo RB et al (2012) Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-kappaB-mediated inflammatory responses. PLoS ONE 7(11):1–11
Wu HK, Sheu SJ (1996) Capillary electrophoretic determination of the constituents of paeoniae radix. J Chromatogr A 753(1):139–146
Jie L et al (2015) Qualitative and quantitative analysis of major constituents of Paeoniae Radix Alba and Paeoniae Radix Rubra by HPLC-DAD-Q-TOF-MS/MS. China J Chin Materia Med 40(9):1762–1770
Kim SH et al (2009) Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J Enzyme Inhib Med Chem 24(5):1138–1140
Yan B et al (2018) Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J Pharm Biomed Anal 160:276–288
Chang-Hong W et al (2008) Comparative pharmacokinetic study of paeoniflorin after oral administration of decoction of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. J Ethnopharmacol 117(3):467–472
Liu ZQ et al (2005) Influence of co-administrated sinomenine on pharmacokinetic fate of paeoniflorin in unrestrained conscious rats. J Ethnopharmacol 99(1):61–67
Cao C et al (2010) Kinetic distribution of paeoniflorin in cortex of normal and cerebral ischemia-reperfusion rats after intravenous administration of Paeoniae radix extract. Biom Chromatogr BMC 20(12):1283–1288
Li YF et al (2011) Pharmacokinetic properties of albiflorin and paeoniflorin after oral administration of pure compound, Radix Paeoniae alba extract and danggui-shaoyao-san extract to rats. J Asian Nat Prod Res 13(2):117–127
Fei F et al (2016) Sensitive analysis and pharmacokinetic study of the isomers paeoniflorin and albiflorin after oral administration of Total Glucosides Of White Paeony Capsule in rats. J Chromatogr B Analyt Technol Biomed Life Sci 1022:30–37
Li Y et al (2016) Pharmacokinetic Comparison of Scutellarin and Paeoniflorin in Sham-Operated and Middle Cerebral Artery Occlusion Ischemia and Reperfusion Injury Rats after Intravenous Administration of Xin-Shao Formula. Molecules 21(9):1–12
Su-Lan H et al (2003) A deglucosylated metabolite of paeoniflorin of the root of Paeonia lactiflora and its pharmacokinetics in rats. Planta Med 69(12):1113–1118
Zhu L et al (2018) Metabolic study of paeoniflorin and total paeony glucosides from Paeoniae Radix Rubra in rats by high-performance liquid chromatography coupled with sequential mass spectrometry. Biomed Chromatogr 32(4):1–13
Gan P et al (2012) Pharmacokinetic comparisons of albiflorin and paeoniflorin after oral administration of Shaoyao-Gancao-Tang and single herb Paeony decoction to rats. Planta Med 78(3):237–243
Longa EZ et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke; a journal of cerebral circulation 20(1):84
Tong L et al (2010) LC-MS/MS determination and pharmacokinetic study of albiflorin and paeoniflorin in rat plasma after oral administration of Radix Paeoniae Alba extract and Tang-Min-Ling-Wan. Biomed Chromatogr 24(12):1324–1331
Luo N et al (2014) Simultaneous determination of bioactive components of Radix Angelicae Sinensis-Radix Paeoniae Alba herb couple in rat plasma and tissues by UPLC-MS/MS and its application to pharmacokinetics and tissue distribution. J Chromatogr B Analyt Technol Biomed Life Sci 963:29–39
Gong C et al (2015) Pharmacokinetic comparisons by UPLC-MS/MS of isomer paeoniflorin and albiflorin after oral administration decoctions of single-herb Radix Paeoniae Alba and Zengmian Yiliu prescription to rats. Biomed Chromatogr 29(3):416–424
Chen M et al (2017) Simultaneous determination of wogonin, oroxylin a, schisandrin, paeoniflorin and emodin in rat serum by HPLC-MS/MS and application to pharmacokinetic studies. Biomed Chromatogr 31(10):e3966
Zhu H et al (2020) Simultaneous determination of ferulic acid, paeoniflorin, and albiflorin in rat plasma by ultra-high performance liquid chromatography with tandem mass spectrometry: Application to a pharmacokinetic study of Danggui-Shaoyao-San. J Sep Sci 43(11):2053–2060
Zhu G et al (2014) Simultaneous Determination of Nine Active Compounds of the Traditional Chinese Medicinal Prescription Shaoyao-Gancao-Tang and Analysis of the Relationship between Therapeutical Effect and Compatibility of Medicines. Evid Based Complement Alternat Med 2014:521038
Zheng L et al (2015) Simultaneous determination of 10 active components in Baizhu Shaoyao San and its single herbs by high-performance liquid chromatography coupled with diode array detection. J Chromatogr Sci 53(4):633–640
Yan Y et al (2014) Simultaneous determination of puerarin, daidzin, daidzein, paeoniflorin, albiflorin, liquiritin and liquiritigenin in rat plasma and its application to a pharmacokinetic study of Ge-Gen Decoction by a liquid chromatography-electrospray ionization-tandem mass spectrometry. J Pharm Biomed Anal 95:76–84
Wu X et al (2017) Simultaneous determination of ginsenoside Rb1, ginsenoside Rg1, paeoniflorin, albiflorin and oxypaeoniflorin in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study of wen-Yang-Huo-Xue soft capsule. Biomed Chromatogr 31(12):e4019
Jeong SH et al (2018) Simultaneous UPLC-MS/MS determination of four components of Socheongryong-tang tablet in human plasma: Application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 1095:214–225
Huang X et al (2014) Simultaneous determination of paeoniflorin, albiflorin, ferulic acid, tetrahydropalmatine, protopine, typhaneoside, senkyunolide I in Beagle dogs plasma by UPLC-MS/MS and its application to a pharmacokinetic study after Oral Administration of Shaofu Zhuyu Decoction. J Chromatogr B Analyt Technol Biomed Life Sci 962:75–81
Jiang F et al (2012) Comparative pharmacokinetic study of paeoniflorin and albiflorin after oral administration of Radix Paeoniae Rubra in normal rats and the acute cholestasis hepatitis rats. Fitoterapia 83(2):415–421
Xu W et al (2016) Enhancement of exposure and reduction of elimination for paeoniflorin or albiflorin via co-administration with total peony glucosides and hypoxic pharmacokinetics comparison. Molecules 21(7):874
He X et al (2004) Effects of cerebral ischemia-reperfusion on pharmacokinetic fate of paeoniflorin after intravenous administration of Paeoniae Radix extract in rats. J Ethnopharmacol 94(2–3):339–344
Xiong S, Wang Y (2016) Simultaneous determination of paeoniflorin from total glucosides of paeony in Sprague-Dawley rats and spontaneously hypertensive rats by high-performance liquid chromatography-tandem mass spectrometry: in vivo and in vitro studies. Biomed Chromatogr 30(11):1766–1771
Acknowledgements
The authors express their thanks for the grants from the National Natural Science Foundation of China under Grant 81403177, the Science and Technology Planning Project of Shenyang Science and Technology Bureau under Grant F12-277-1-14, and the Innovative Talents Support Program of Liaoning Province under Grant LR2018047.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LZ, SS, YH, YL and XL. The manuscript was written and proved by LZ, YL, XL and YL. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study has been approved by the ethics committee for research animals of Liaoning University, and all experimental operations were in accordance with the welfare of animals.
Informed Consent
All authors have made substantial contributions to this research. And all authors have drafted the work or revised it critically for important intellectual content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, L., Sun, S., Li, X. et al. Comparative Pharmacokinetic Studies of Paeoniflorin, Albiflorin and Oxypaeoniflorin Between Normal and Cerebral Ischemia–Reperfusion Rats Based on the Simultaneous Determinations Using an UPLC-MS–MS Method. Chromatographia 83, 1345–1354 (2020). https://doi.org/10.1007/s10337-020-03954-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03954-4