Abstract
The thermodynamic surface and interfacial properties of some hydrocarbons used as process oils in rubber industry were determined using the inverse gas chromatography technique at infinite dilution. In this paper, four different common process oils, such as distillated aromatic extract, treated distillate aromatic extract, mildly extracted solvate, and hydro-processed naphthenic oil, were studied. An important effect of the temperature on the dispersive component of the surface energy of different hydrocarbons was proved. These hydrocarbon materials exhibit a strong dependency of their surface acid–base properties on the temperature. The specific surface enthalpy and entropy, as well as the acid–base constants KA, KD and the amphoteric constant K of hydrocarbon surfaces, strongly depend on the temperature. It was proved that the hydro-processed naphthenic oil presents the highest acidity relative to other hydrocarbon materials.
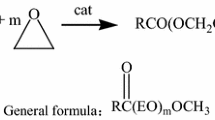
Graphic abstract
















Similar content being viewed by others
References
Santos JMRCA, Guthrie JT (2005) Analysis of interactions in multicomponent polymeric systems: the key-role of inverse gas chromatography. Mater Sci Eng R 50:79–107
Gamelas JAF (2013) The surface properties of cellulose and lignocellulosic materials assessed by inverse gas chromatography: a review. Cellulose 20:2675–2693
Mukhopadhyay P, Schreiber HP (1995) Aspects of acid-base interactions and use of inverse gas chromatography. Colloids Surf A 100:47–71
Gamelas JAF, Ferraz E, Rocha F (2014) An insight into the surface properties of calcined kaolinitic clays: the grinding effect. Colloids Surf A 455:49–57
Ansari DM, Price GJ (2004) Polymer chromatographic estimation of filler surface energies and correlation with photodegradation of kaolin filled polyethylene. Polymer 45:1823–1831
Fekete E, Móczo J, Pukánszky B (2004) Determination of the surface characteristics of particulate fillers by inverse gas chromatography at infinite dilution: a critical approach. J Colloid Interface Sci 269:143–152
Ward TC, Lloyd DR, Schreiber HP (eds), Inverse gas chromatography, ACS Symp Ser. No. 391, Washington, DC, 1989.
Cline D, Dalby R (2002) Predicting the quality of powders for inhalation from surface energy and area. Pharm Res 19:1274–1277
Feeley JC, York P, Sumby BS, Dicks H (1998) Processing effects on the surface properties of α-lactose monohydrate assessed by inverse gas chromatography (IGC). Int J Pharm 172:89
M. D. Ticehurst, Characterisation of the surface energetics of pharmaceutical powders by inverse gas chromatography. University of Bradford, York, Ph.D. thesis, 1995.
Buckton G (1997) Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Adv Drug Deliv Rev 26:17–27
Al-Ghamdi A, Al-Saigh ZY (2002) Surface and thermodynamic characterization of conducting polymers by inverse gas chromatography. I Polyaniline J Chromatogr A 969:229–243
Que R, Wu D, Al-Saigh ZY (2007) Surface and thermodynamic characterization of conducting polymers by inverse gas chromatography: II. Polyaniline and its blend. J Chromatogr A 1146:93–102
Boukerma K, Mičušík M, Mravčáková M, Omastova M, Vaulay MJ, Beaunier P et al (2007) Surfactant-assisted control of the surface energy and interfacial molecular interactions of polypyrrole. Coll Surf A 293:28–38
Bailey RA, Persaud KC (1998) Application of inverse gas chromatography to characterisation of a polypyrrole surface. Anal Chim Acta 363:147–156
Surana R, Randall L, Pyne A, Vemuri NM, Suryanarayanan R (2003) Determination of glass transition temperature and in situ study of the plasticizing effect of water by inverse gas chromatography. Pharm Res 20:1647–1654
Baoli S, Qianru Z, Lina J, Yang L, Bin L (2007) Surface Lewis acid–base properties of polymers measured by inverse gas chromatography. J Chromatogr A 1149:390–393
Santos J, Gil H, Portugal A, Guthrie JT (2001) Characterisation of the surface of a cellulosic multi-purpose office paper by inverse gas chromatography. Cellulose 8:217–224
Voelkel A, Grzeskowiak T (2000) The use of solubility parameters in characterization of titanate modified silica gel by inverse gas chromatography. Chromatographia 51:608–614
Newell E, Buckton G, Butler DA, Thielmann F, Williams DR (2001) The use of inverse phase gas chromatography to measure the surface energy of crystalline, amorphous, and recently milled lactose. Pharm Res 18:662–666
Kalantzopoulou FR, Artemiacti T, Bassiotis I, Katsanos NA, Plagianakos V (2001) Time separation of adsorption sites on heterogeneous surfaces by inverse gas chromatography. Chromatographia 53:315–320
Reutenauer F (2003) Thielmann, the interaction of cotton fabrics and the interaction with perfume molecules by inverse gas chromatography (IGC). J Mater Sci 38:2205–2208
Chow AHL, Tong HHY, Shekunov BY, York P (2004) Letter to editor: use of inverse gas chromatography (IGC) to determine the surface energy and surface area of powdered materials. Pharma Res 21(9):1718–1720
Askin A, Yazici DT (2008) A study of the surface analysis of some water-soluble polymers by inverse gas chromatography. Surf Interface Anal 40:1237–1241
Sreekanth TVM, Reddy KS (2007) Analysis of Solvent–Solvent Interactions in mixed isosteric solvents by inverse gas chromatography. Chromatographia 65:326–330
Yang YC, Yoon PR (2007) Examination of the surface properties of kaolinites by inverse gas chromatography: acid-base properties. Korean J Chem Eng 24:451–456. https://doi.org/10.1007/s11814-007-0078-7
Przybyszewska M, Krzywania A, Zaborski M, Szynkowska MI (2009) Surface properties of zinc oxide nanoparticles studied by inverse gas chromatography. J Chromatogr A 1216(27):5284–5291
Hamieh T, Rageul-Lescouet M, Nardin M, Haidara H, Schultz J (1997) Study of acid-base interactions between some metallic oxides and model organic molecules. Coll Surf A 125:155–161
Hamieh T, Rezzaki M, Schultz J (2001) Study of the transition temperatures and acid-base properties of poly (methyl methacrylate) adsorbed on alumina and silica, by using inverse gas chromatography technique. Coll Surf A 189(1–3):279–291
Hamieh T (2011) Determination of the transition phenomena of poly(α-n-alkyl) methacrylates adsorbed on silica by inverse gas chromatography (IGC). J Polym Res 18:1159–1168
Hamieh T (2011) New approach for the determination of acid-base properties of poly(α-n-alkyl) methacrylates adsorbed on silica by inverse gas chromatography (IGC). Chromatographia 73(7–8):709–719
Mohammadi-Jam S, Waters KE (2014) Inverse gas chromatography applications: a review. Adv Coll Interf Sci 212:21–44
Saint Flour C, Papirer E (1982) Gas-solid chromatography A method of measuring surface free energy characteristics of short glass fibers. 1. Through adsorption isotherms. Ind Eng Chem Prod Res Dev 21:337–341
Saint Flour C, Papirer E (1982) Gas-solid chromatography: method of measuring surface free energy characteristics of short fibers. 2. Through retention volumes measured near zero surface coverage. Ind Eng Chem Prod Res Dev 21:666–669
Hamieh T, Toufaily T, Mouneimné AB (2011) Effect of the tacticity of PMMA adsorbed on alumina and silica on the specific entropy change of polymer by inverse gas chromatography. Chromatographia 73(1–2):99–107
Hamieh T, Schultz J (2002) New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution. J Chromatogr A 969(1–2):17–47
Conder JR, Young CL (1979) Physical measurements by gas chromatography. Wiley, New York
Van Alsten JG, Sauer BB, Walsh J (1992) Polymer dynamics at the melt/solid interface: experimental evidence of reduced center of mass mobility. Macromolecules 25:4046–4048
Siffert B, Kuczinski J, Papirer E (1990) Relationship between electrical charge and flocculation of heavy oil distillation residues in organic medium. J Coll Interf Sci 135(1):107–117
Schultz J, Lavielle L, Martin C (1987) The role of the interface in carbon fibre-epoxy composites. J Adhes 23(1):45–60
Ansari DM, Price GJ (2004) Correlation of mechanical properties of clay filled polyamide mouldings with chromatographically measured surface energies. Polymer 45:3663–3670
Przybyszewska M, Krzywania A, Zaborski M, Szynkowska MI (2009) Surface properties of zinc oxide nanoparticles studied by inverse gas chromatography. J Chromatogr A 1216:5284–5291
James AT, Martin JP (1952) Gas-liquid partition chromatography: the separation and microestimation of volatile fatty acids from formic acid to dodecanoic acid. Biochem 50:679–690
Gutmann V (1978) The donor-acceptor approach to molecular interactions. Plenum, New York
Hamieh T, Rageul-Lescouet M, Nardin M, Rezzaki M, Schultz J (1997) Etude des interactions spécifiques entre certains oxydes métalliques et des molécules organiques modèles. J Chim Phys 94:503–524
Riddle FL, Fowkes FM (1990) Spectral shifts in acid-base chemistry. Van der Waals contributions to acceptor numbers, spectral shifts in acid-base chemistry. 1. Van der Waals contributions to acceptor numbers. J Am Chem Soc 112(9):3259–3264
Farshchi N, Abbasian A (2017) Inverse gas chromatography study of Hansen solubility parameters of rubber process oils (DAE, TDAE, MES and NAP). Rubber Chem Technol. https://doi.org/10.5254/rct.19.83697
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Research involving human participants and/or animals
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamieh, T., Abbasian, A. & Farshchi, N. New Methods to Characterize the Surface and Interface Acid–Base Properties of Some Hydrocarbons by Inverse Gas Chromatography. Chromatographia 83, 615–629 (2020). https://doi.org/10.1007/s10337-020-03878-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03878-z