Abstract
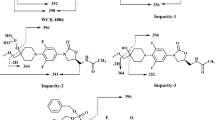
Zidebactam (ZID) is a novel drug in clinical development; it potentiates the antibacterial activity of β-lactam antibiotics. Two reversed phase liquid chromatographic (RP-LC) methods were developed for ZID; a mass spectrometer (MS) compatible method for identification and another UV detection method for the quantification of impurities. Four impurities and three degradation products (DPs) were identified using LC–MS/MS. Both methods were developed on Hydrosphere C18 stationary phase which was found to be unique for the purpose due to its hydrophilic surface. The chromatographic separation of ZID and impurities was achieved in gradient mode using mobile phase consisting of ammonium formate in LC–MS/MS studies and ammonium dihydrogen phosphate in UV method. Acetonitrile (ACN) was employed as an organic solvent. ZID was subjected to hydrolytic, oxidative, photolytic and thermal stress conditions. The degradation was observed in acidic, basic and oxidative conditions. The UV method was validated. The method was accurate and precise to quantify impurities; accuracy was greater than 99.8% with precision of less than 4%. The method was highly sensitive to detect impurities as low as 1.4 ng on column. The developed method was employed for quality control and stability studies of the new drug substance during pre-clinical and clinical studies. The same method was also adapted for analysis of a parenteral drug product.
Graphic abstract










Similar content being viewed by others

Change history
08 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10337-022-04142-2
References
Joshi SN, Wankhede KS, Jadhav SB, Pawar SS, Ahirrao VK, Bhawsar SB, Deshpande PK, Yeole RD, Patel MV (2013) A process for preparation of (2S,5R)-7-Oxo-6-sulphooxy-2-[Nʹ-((3R)-piperidin-3-carbonyl)-hydrazinocarbonyl]-1,6-diaza- bicyclo[3.2.1]octane. World Intellectual Property Organization: International application published under the patent cooperation treaty (PCT), Publication Number WO2014/135931
Patel MV, Deshpande PK, Bhawsar SB, Bhagwat SS, Jafri MA, Mishra A, Pavase L, Gupta S, Kale R, Joshi SN (2011) 1,6-diaza- bicyclo[3.2.1]octane-7-one derivatives and their use in treatment of bacterial infections. World Intellectual Property Organization: International application published under the patent cooperation treaty (PCT), Publication Number US2014/0148431A1
Sader HS, Castanheira M, Huband M, Jones RN, Flamm RK (2017) WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative bacteria collected worldwide in 2015. Antimicrob Agents Chemother 61(5):e00072-17. https://doi.org/10.1128/AAC.00072-17
Sader HS, Rhomberg PR, Flamm RK, Jones RN, Castanheira M (2017) WCK 5222 (cefepime-zidebactam) antimicrobial activity against clinical isolates of Gram-negative organisms producing clinically relevant β-lactamases. J Antimicrob Chemother 72(6):1696–1703. https://doi.org/10.1093/jac/dkx050
Livermore DM, Mushtaq S, Warner M, Vickers A, Woodford N (2017) In vitro activity of cefepime/zidebactam (WCK 5222) against gramnegative bacteria. J Antimicrob Chemother 56(5):1373–1385. https://doi.org/10.1093/jac/dkw593
Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonoma RA, Oliver A (2017) WCK 5107 (zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “β-lactam enhancer” activity against Pseudomonas aeruginosa, including multidrug-resistant metallo-β-lactamase-producing high-risk clones. Antimicrob Agents Chemother 61(6):e02529-16. https://doi.org/10.1128/AAC.02529-16
Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonoma RA, Oliver A (2017) Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinebacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother 61(11):e01238-17. https://doi.org/10.1128/AAC.01238-17
Rawat T, Pandey IP (2015) Forced degradation studies for drug substance and drug products—scientific and regulatory considerations. J Pharm Sci Res 7(5):238–241
ICH (2002) M4Q (R1) International conference on harmonisation tripartite guideline. The common technical document for the registration of pharmaceuticals for human use: quality. http://www.ich.org
ICH (2006) Q3A (R2) Impurities in new drug substance. In: International conference on harmonisation tripartite guideline. The common technical document for the registration of pharmaceuticals for human use. http://www.ich.org
ICH (1999) Q6A specifications: Test procedures and acceptance, criteria for new drug substances and new drug products: chemical substances. In: International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. http://www.ich.org
Patil KR, Tambe H, Zope V, Chavan RP, Yeole RD, Patel MV (2018) Simultaneous determination of zidebactam and cefepime in dog plasma by LC–MS.MS and its application to pre-clinical pharmacokinetic study. Biomed Chromatog 32(8):e4249. https://doi.org/10.1002/bmc.4249
Suryawanshi G, Bandal R, Harole M, Pise K (2016) A validated stability indicating RP-HPLC method for simultaneous determination of avibactam and ceftazidime in bulk and pharmaceutical dosage form. World J Pharmacy Pharm Sci 5(7):1611–1621
Shaikh MN, Sadath A (2015) RP-HPLC method development and validation for the simultaneous estimation of ceftazidime and avibactam in intravenous infusion. Int J Eng Tech Sci 2(11):1–5
Sillen H, Mitchell R, Sleigh R, Mainwaring G, Catton K, Houghton R, Glending K (2015) Determination of avibactam and ceftazidime in human plasma samples by LC–MS. Bioanal 7(12):1423–1434
Rizk ML, Rhee EG, Jumes PA, Gotfried MH, Zhao T, Mangin E, Sheng Bi, Cynthia M, Eng C, Zhang Z, Butterton JR (2018) Intrapulmonary pharmacokinetics of relebactam, a novel β-lactamase inhibitor, dosed in combination with imipenem/cilastatin in healthy subjects. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01411-17
ICH (2005) Q2R1 Validation of analytical procedure text and methodology. In: International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. http://www.ich.org
ICH (1996) Q1B Stability testing: photo-stability testing of new drug substances and products. In: International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. http://www.ich.org
http://www.ymc.co.jp/en/columns/hydrosphere_c18/index.html. Accessed 2011
Acknowledgements
Authors thank the management of Wockhardt Limited for supporting this work.
Funding
This study is part of Wockhardt Ltd in house drug discovery programe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding publication of this manuscript.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rane, V.P., Ahirrao, V.K., Patil, K.R. et al. Impurity Profiling of a Novel β-Lactam Enhancer: Zidebactam. Chromatographia 83, 423–437 (2020). https://doi.org/10.1007/s10337-019-03845-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03845-3