Abstract
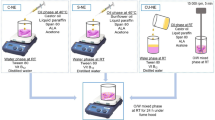
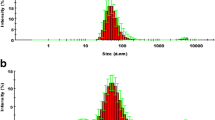
Oncocalyxone A (onco A) isolated from heartwood of Cordia oncocalyx (Auxemma oncocalyx), exhibits antitumor, analgesic and anti-inflammatory activities. In order to produce drug delivery systems containing onco A, the objective of this study was to develop and validate an HPLC method for quantifying onco A in different media and nanocapsules. The validation method was performed according to ICH guidelines for pharmaceutical products. The solubility of onco A was determined in deionized water, simulated gastric and intestinal fluids, buffer solutions at pH 5.5, 7.0, 9.0 and oils: copaiba, soybean and medium-chain triglycerides. The partition coefficients of onco A between n-octanol, oils and aqueous phase were determined at 25 °C. Accuracy, intra- and inter-day precision values presented low random errors (less than or equal to 2.53, 2.21 and 3.12%, respectively). Onco A solubility was greater in water, buffer solutions, and simulated gastric and intestinal fluids (817 ± 54 μg mL−1) than in oils (434 ± 25 μg mL−1). Onco A showed hydrophilic characteristics (log P < 1). The onco A content in chitosan-coated nanoparticles was 99.6% (995.8 ± 26.5 μg mL−1). In conclusion, a robust HPLC method for quantifying onco A was successfully developed, validated and applied for determining onco A solubility in different pHs, partition coefficient and nanocapsules.




Similar content being viewed by others
References
Abraham I, Josh R, Pardasani P, Pardasani RT (2011) Recent advances in 1,4-benzoquinone, chemistry. J Braz Chem Soc 22:385–421
Gottschling M, Miller JS (2006) Classification of the taxonomic position of Auxemma, Patagonula, and Saccellium (Cordiaceae, Boraginales). Syst Bot 3:361–367
Ferreira MAD, Nascimento NRF, Souza CM, Pessoa ODL, Lemos TLG, Ventura JS, Schattner M, Chudzinski-Tavassi AM (2008) Oncocalyxone A inhibits human platelet aggregation by increasing cGMP and by binding to GP Iba glycoprotein. Br J Pharmacol 154:1216–1224
Viana SM, Ferreira MAD, Guerra PV, Viana GSB, Teixeira JM (2015) In vitro and in vivo evaluation of quinones from Auxemma Oncocalix Taub. On Leishmania Brasiliensis. J Med Plants Res 9:132–139
Ilayaraja S, Prabakaran K, Manivannan R (2014) Evaluation of anti-bacterial, analgesic and anti-inflammatory activities of Oncocalyxone A isolated from Prenanthes sarmentosus. J Appl Pharm Sci 4:88–91
Sivagnanam I, Kalaivanam P, Rajamanickam M (2013) Antibiabetic activity of oncocalyxone A isolated from Prenanthes sarmenthosus. Int J Pharm Sci 5:630–633
Levya A, Pessoa C, Boogaerdt F, Sokaroski R, Lemos TL, Weltmore LA, Hurura RR, Moraes MO (2000) Oncocalyxones A and C, 1,4-anthracenediones from Auxemma oncocalyx: comparison with anticancer 1,9-anthracenediones. Anticancer Res 20:1029–1032
Di L, Kerns EH, Carter GT (2009) Drug-like property concepts in pharmaceutical design. Curr Pharm Des 15:2184–2194
Sharapova A, Ol’Khovich M, Blokhina S, Perlovich G (2017) Physico-chemical characterization antituberculosis thioacetazone: vapor pressure, solubility and lipophilicity. J Chem Thermodyn 108:18–25
Wolfender JL (2009) HPLC in natural product analysis: the detection issue. Planta Med 75:719–734
Costa JGM, Arriaga AM, Mattos CMC, Pessoa ODL, Nascimento Lemos TLG (2004) High-performance liquid chromatographic analysis of bioactive quinones from Auxemma glazioviana. Arkivoc 6:72–79
International Conference on Harmonization (ICH) (2005) Q2 (R1): validation of analytical procedures: text and methodology, November 2005. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed 14 Mar 2019
Food and Drug Administration (FDA) (2015) Analytical procedures and methods validation for drugs and biologics: guidance for industry, 2015. https://www.fda.gov/downloads/drugs/guidances/ucm386366.pdf. Accessed 14 Mar 2019
Agência Nacional de Vigilância Sanitária (ANVISA) RDC N°166-24 July 2017. http://portal.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401. Accessed 14 Mar 2019
Pessoa ODL, Lemos TL, Silveira ER, Raimundo BF (1993) Novel cordiachromes isolated from Auxemma oncocalyx. Nat Prod Lett 2:145–150
Pessoa ODL, Lemos TL, Carvalho MG, Braz-Filho R (1995) Cordiachromes from Auxemma oncocalyx. Phytochemistry 40:1777–1786
Barreto ACH, Santiago VR, Freire RM, Mazetto SE, Denardin JC, Mele G, Cavalvante IM, Ribeiro MENP, Ricardo NMPS, Gonçalves T, Carboni L, Lemos TLG, Pessoa ODL, Fechine PBA (2013) Magnetic nanosystem for cancer therapy using oncocalyxone A, an antitumor secondary metabolite isolated from a Brazilian plant. Int J Mol Sci 14:18269–18283
United States of Pharmacopoeia (USP) (2016) 39th edition, Rockville. https://www.uspnf.com. Accessed 14 Mar 2019
Xavier-Junior FH, Gueutin C, Morais ARV, Alencar EN, Egito EST, Vauthier C (2016) HPLC method for the dosage of paclitaxel in copaiba oil: development, validation, application to the determination of the solubility and partition coefficients. Chromatographia 79:405–412
Bender EA, Adornec MD, Colomé LM, Abdalla DSP, Guterres SS, Pohlmann AR (2012) Hemocompatibility of poly-ε-caprolactone lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm 426:271–279
Moein MM, El Beqqali A, Abdel-Rehim M (2017) Bioanalytical method development and validation: critical concepts and strategies. J Chromatogr B 1043:3–11
Sahu PK, Ramisetti NR, Cecchi T, Swain S, Patro CS, Jagadeesh P (2018) An overview of experimental designs in HPLC method development and validation. J Pharm Biomed Anal 147:590–611
Bertolucci SKV, Pereira ABD, Pinto JEBP, Ribeiro JAA, Oliveira AB, Braga FC (2009) Development and validation of an RP-HPLC method for quantification of cinnamic acid derivatives and kaurane-type diterpenes in Mikania laevigata and Mikania glomerata. Planta Med 75:280–285
Cheng CL, Shalabh Garg G (2014) Coefficient of determination for multiple measurement error models. J Multivar Anal 126:137–152
Xu L, Jiang Y, Qiu R (2018) Parametric study and global sensitivity analysis for co-pyrolysis of rape straw and waste tire via variance-based decomposition. Bioresour Technol 247:545–552
Mcnair H, Polite LN (2007) 17 Troubleshooting in high performance liquid chromatography. Sep Sci Technol 8:459–477
Jbeyli M, Kreesler J (2017) Fluorophilicity and lipophilicity of fluorinated rhodamines determined by their partition coefficients in biphasic solvent systems. J Fluor Chem 193:67–72
Bannan CC, Calabró G, Kyu DY, Mobley DL (2016) Calculating partition coefficients of small molecules in octanol/water and cyclohexane/water. J Chem Theory Comput 12:4015–4024
Xavier-Júnior FH, Egito EST, Morais ARV, Alencar EN, Maciuk A, Vauthier C (2018) Experimental design approach applied to the development of chitosan coated poly(isobutylcyanoacrylate) nanocapsules encapsulating copaiba oil. Colloids Surf A Physicochem Eng Asp 536:251–258
Acknowledgements
The authors thank the Brazilian research agencies for their financial support: Brazilian Ministry of Education (CAPES, PNPD-PPGNANOFARMA), Brazilian Council for Research and Development (CNPq, Grant #311232/2013-2), Brazilian Ministry of Science and Technology (MCTI), Laboratórios Associados em Nanotecnologia-UFPE (LARnano/SisNANO-MCTI, Grant # 402282/2013-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavares, C.A., Xavier-Júnior, F.H., Pessoa, O.D.L. et al. Validation of an HPLC–UV Method for Quantifying Oncocalyxone A in Different Media and Nanocapsules. Chromatographia 82, 809–818 (2019). https://doi.org/10.1007/s10337-019-03716-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03716-x