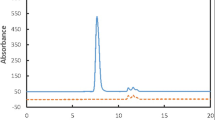
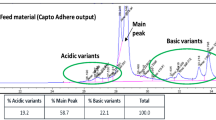
Abstract
Ion exchange chromatography is widely used for charge variant analysis of proteins, including monoclonal antibodies. In this study, a simple and robust salt gradient cation exchange chromatography was developed and validated for quantitative determination of cetuximab in biopharmaceutical formulations. For this purpose, we investigated the effect of various parameters including buffer composition, column temperature, pH, gradient volume and flow rate on chromatographic separation of charge variants to achieve the acceptable peak separation, and the optimum condition was selected. Validation of the method was done in accordance with the International Conference on Harmonization (ICH) guidelines. The developed method was found to provide a linear regression over the concentration range of 0.06–2.00 mg mL−1 yielding a correlation coefficient of 0.9972. The limits of detection and quantification for the developed method were 0.02 and 0.06 mg mL−1, respectively. The intra-day and inter-day precision had relative standard deviation values ≤ 2.7%. The robustness of the method was assessed by changes in the applied pH range of buffer, temperature, mobile phase composition, and flow rate. Specificity of the method was confirmed by evaluation of baseline resolution of the mAb variants from product excipients, which showed no interference between excipients and cetuximab. The stability indicating capability of this method was determined using photodegraded, and mechanically and thermally stressed samples. The proposed method could be applied as a simple, precise, and robust quantitative technique which can be reproduced in any labs for the high-throughput quality control and stability assessment of in-process and final product samples.




Similar content being viewed by others
References
Beck A, Sanglier-Cianférani S, Van Dorsselaer A (2012) Biosimilar, biobetter, and next generation antibody characterization by mass spectrometry. ACS Publications, Washington
Nicolaides NC, Sass PM, Grasso L (2006) Monoclonal antibodies: a morphing landscape for therapeutics. Drug Dev Res 67(10):781–789
Rea JC, Moreno GT, Lou Y, Farnan D (2011) Validation of a pH gradient-based ion-exchange chromatography method for high-resolution monoclonal antibody charge variant separations. J Pharm Biomed Anal 54(2):317–323
Fekete S, Gassner A-L, Rudaz S, Schappler J, Guillarme D (2013) Analytical strategies for the characterization of therapeutic monoclonal antibodies. TrAC Trends Anal Chem 42:74–83
Beck A, Wagner-Rousset E, Bussat M-C, Lokteff M, Klinguer-Hamour C, Haeuw J-F, Goetsch L, Wurch T, Dorsselaer AV, Corvaïa N (2008) Trends in glycosylation, glycoanalysis and glycoengineering of therapeutic antibodies and Fc-fusion proteins. Curr Pharm Biotechnol 9(6):482–501
Vlasak J, Ionescu R (2008) Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Curr Pharm Biotechnol 9(6):468–481
Khawli LA, Goswami S, Hutchinson R, Kwong ZW, Yang J, Wang X, Yao Z, Sreedhara A, Cano T, Tesar DB (2010) Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs 2(6):613–624
Tang L, Sundaram S, Zhang J, Carlson P, Matathia A, Parekh B, Zhou Q, Hsieh M-C (2013) Conformational characterization of the charge variants of a human IgG1 monoclonal antibody using H/D exchange mass spectrometry. MAbs 5(1):114–125
Flatman S, Alam I, Gerard J, Mussa N (2007) Process analytics for purification of monoclonal antibodies. J Chromatogr B 848(1):79–87
Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J (2008) Heterogeneity of monoclonal antibodies. J Pharm Sci 97(7):2426–2447
Fekete S, Beck A, Fekete J, Guillarme D (2015) Method development for the separation of monoclonal antibody charge variants in cation exchange chromatography, part II: pH gradient approach. J Pharm Biomed Anal 102:282–289
Talebi M, Nordborg A, Gaspar A, Lacher NA, Wang Q, He XZ, Haddad PR, Hilder EF (2013) Charge heterogeneity profiling of monoclonal antibodies using low ionic strength ion-exchange chromatography and well-controlled pH gradients on monolithic columns. J Chromatogr A 1317:148–154
Fekete S, Beck A, Veuthey J-L, Guillarme D (2015) Ion-exchange chromatography for the characterization of biopharmaceuticals. J Pharm Biomed Anal 113:43–55
Urmann M, Graalfs H, Joehnck M, Jacob LR, Frech C (2010) Cation-exchange chromatography of monoclonal antibodies: Characterisation of a novel stationary phase designed for production-scale purification. MAbs 2(4):395–404
Giacometti J, Josić D (2013) Protein and peptide separations. In: Fanali S, Haddad PR, Poole CF, Lloyd D (eds) Liquid chromatography, 1 edn. Elsevier, New York, pp 149–184
Müller-Späth T, Aumann L, Melter L, Ströhlein G, Morbidelli M (2008) Chromatographic separation of three monoclonal antibody variants using multicolumn countercurrent solvent gradient purification (MCSGP). Biotechnol Bioeng 100(6):1166–1177
Subramanian G (2012) Biopharmaceutical production technology, 2 volume set, vol 1. Wiley, New York
Farnan D, Moreno GT (2009) Multiproduct high-resolution monoclonal antibody charge variant separations by pH gradient ion-exchange chromatography. Anal Chem 81(21):8846–8857
Rozhkova A (2009) Quantitative analysis of monoclonal antibodies by cation-exchange chromatofocusing. J Chromatogr A 1216(32):5989–5994
Shan L, Anderson DJ (2002) Gradient chromatofocusing. Versatile pH gradient separation of proteins in ion-exchange HPLC: characterization studies. Anal Chem 74(21):5641–5649
Martínez-Ortega A, Herrera A, Salmerón-García A, Cabeza J, Cuadros-Rodríguez L, Navas N (2016) Study and ICH validation of a reverse-phase liquid chromatographic method for the quantification of the intact monoclonal antibody cetuximab. J Pharm Anal 6(2):117–124
Sundaram S, Matathia A, Qian J, Zhang J, Hsieh M-C, Liu T, Crowley R, Parekh B, Zhou Q (2011) An innovative approach for the characterization of the isoforms of a monoclonal antibody product. MAbs 3(6):505–512
Ayoub D, Jabs W, Resemann A, Evers W, Evans C, Main L, Baessmann C, Wagner-Rousset E, Suckau D, Beck A (2013) Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. MAbs 5(5):699–710
Müller-Späth T, Krättli M, Aumann L, Ströhlein G, Morbidelli M (2010) Increasing the activity of monoclonal antibody therapeutics by continuous chromatography (MCSGP). Biotechnol Bioeng 107(4):652–662
Hintersteiner B, Lingg N, Janzek E, Mutschlechner O, Loibner H, Jungbauer A (2016) Microheterogeneity of therapeutic monoclonal antibodies is governed by changes in the surface charge of the protein. Biotechnol J 11(12):1617–1627
Fekete S, Beck A, Fekete J, Guillarme D (2015) Method development for the separation of monoclonal antibody charge variants in cation exchange chromatography, part I: salt gradient approach. J Pharm Biomed Anal 102:33–44
Konieczka P (2007) The role of and the place of method validation in the quality assurance and quality control (QA/QC) system. Crit Rev Anal Chem 37(3):173–190
Guideline IHT (2005) Validation of analytical procedures: text and methodology Q2 (R1). In: International conference on harmonization, Geneva, Switzerland, pp 11–12
Bakshi M, Singh S (2002) Development of validated stability-indicating assay methods—critical review. J Pharm Biomed Anal 28(6):1011–1040
Guidance for industry: Analytical procedures and methods validation for drugs and biologics, US Department of Health and Human Services (2014). https://www.fda.gov/downloads/drugs/guidances/ucm386366.pdf. Accessed July 2018
ICH: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Topic Q1B: Stability testing: photostability testing of new drug substances and products (1996). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1B/Step4/Q1B_Guideline.pdf. Accessed July 2018
Biacchi M, Gahoual R, Said N, Beck A, Leize-Wagner E, François Y-N (2015) Glycoform separation and characterization of cetuximab variants by middle-up off-line capillary zone electrophoresis-UV/electrospray ionization-MS. Anal Chem 87(12):6240–6250
ICH: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Topic Q5C: Stability testing of biotechnological/biological products (1995). http://www.ich.org/fleadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5C/Step4/Q5C_Guideline.pdf. Accessed July 2018
Tonnesen HH (2004) Photostability of drugs and drug formulations. CRC Press, Boca Raton
Wei Z, Feng J, Lin H-Y, Mullapudi S, Bishop E, Tous GI, Casas-Finet J, Hakki F, Strouse R, Schenerman MA (2007) Identification of a single tryptophan residue as critical for binding activity in a humanized monoclonal antibody against respiratory syncytial virus. Anal Chem 79(7):2797–2805
Pattison DI, Rahmanto AS, Davies MJ (2012) Photo-oxidation of proteins. Photochem Photobiol Sci 11(1):38–53
Kerwin BA, Remmele RL (2007) Protect from light: photodegradation and protein biologics. J Pharm Sci 96(6):1468–1479
Vanhooren A, Devreese B, Vanhee K, Van Beeumen J, Hanssens I (2002) Photoexcitation of tryptophan groups induces reduction of two disulfide bonds in goat α-lactalbumin. Biochemistry 41(36):11035–11043
Acknowledgements
This work is a part of A. farjami thesis, submitted for Ph.D. degree (no. 117) and supported by Research Council, Tabriz University of Medical Sciences and we would like to thank the CinnaGen Medical Biotechnology Center for kindly providing all of the cetuximab medicinal samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Farjami, A., Siahi-Shadbad, M., Akbarzadehlaleh, P. et al. Development and Validation of Salt Gradient CEX Chromatography Method for Charge Variants Separation and Quantitative Analysis of the IgG mAb-Cetuximab. Chromatographia 81, 1649–1660 (2018). https://doi.org/10.1007/s10337-018-3627-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3627-9