Abstract
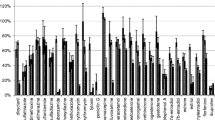
Steroid hormones, mainly secreted by vertebrates, are discharged into the soil environment through surface runoff and land application of animal manure, sewage sludge, and organic fertilizers. The adequate analytical methods for steroid hormones in soil are lacking due to the requirement of rigorous sample pre-treatment. In this study, a rapid and effective modified QuEChERS (quick, easy, cheap, effective, rugged, and safe) method was developed for trace simultaneous analysis of 12 steroid hormones (estrogen, androgens, and progestogens) in soil samples using ultra performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS). Samples were extracted by the mixture of acetonitrile and acetate buffer, and then cleaned with PSA/C18 absorbents. The QuEChERS method was validated by evaluating the linearity, repeatability, accuracy and precision. A suitable linear relationship was obtained in the mass concentration range of 1–100 ng g−1 with high correlation coefficients (> 0.9927). The method enabled the determination of the target analytes with limits of detection between 0.0014 and 0.462 ng g−1 and limits of quantification between 0.0047 and 1.54 ng g−1. Soil was spiked at 5, 50 and 100 ng g−1, and the recoveries ranged from 75.17 to 110.33% with relative standard deviations ≤ 9.45. The developed method was successfully applied to the analysis of soil samples collected in Beijing, and five hormones (E1, E3, αE2, And, and Tes) were detected with the concentrations ranging from 0.35 to 7.09 ng g−1.
Graphical Abstract






Similar content being viewed by others
References
Shappell NW, Hyndman KM, Bartell SE et al (2010) Comparative biological effects and potency of 17α-and 17β-estradiol in fathead minnows. Aquat Toxicol 100(1):1–8. https://doi.org/10.1016/j.aquatox.2010.07.005 (Epub 2010 Jul 7)
Gibson R, Smith MD, Spary CJ et al (2005) Mixtures of Estrogenic contaminants in bile of fish exposed to wastewater treatment works effluents. Environ Sci Technol 39(8):2461–2471. https://doi.org/10.1021/es048892g
Alexandre B, Emmanuelle V (2015) Development of a method for the analysis of hormones and pharmaceuticals in earthworms by quick, easy, cheap, effective, rugged and safe (QuEChERS) extraction followed by liquid chromatography–tandem mass spectrometry (LC–MS/MS). Anal Bioanal Chem 407(26):7995–8008. https://doi.org/10.1007/s00216-015-8972-z
Khanal SK, Xie B, Thompson ML et al (2006) Fate, transport and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol 40(21):6537–6546. https://doi.org/10.1021/es0607739
Maier RM, Pepper LI, Gerba CP (2000) Environmental microbiology. Academic Press, New York, pp 61–80
Kuster M, Azevedo DA, López de Alda MJ (2009) Analysis of phytoestrogens, progestogens and estrogens in environmental waters from Rio de Janeiro (Brazil). Environ Int 35(7):997–1003. https://doi.org/10.1016/j.envint.2009.04.006
Ternes TA, Andersen H, Gilberg D et al (2002) Determination of estrogens in sludge and sediments by liquid extraction and GC/MS/MS. Anal Chem 74(14):3498–3504. https://doi.org/10.1021/ac015717z
Kumar K, Gupta SC, Chander Y, Singh AK (2005) Antibiotic use in agriculture and its impact on the terrestrial environment. Adv Agron 87:1–54. https://doi.org/10.1016/S0065-2113(05),87001-4
Nieto A, Borrull F, Pocurull E, Marcé RM (2008) Determination of natural and synthetic estrogens and their conjugates in sewage sludge by pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1213(2):224–230. https://doi.org/10.1016/j.chroma.2008.10.043
Petrović M, Hernando MD, Barceló D et al (2005) Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chromatogr A 1067:1–14. https://doi.org/10.1016/j.chroma.2004.10.110
Vulliet E, Wiest L, Baudot R et al (2008) Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1210(1):84–89. https://doi.org/10.1016/j.chroma.2008.09.034 (Epub 2008 Sep 16)
Kot Wasik A, Debska J, Namiesnik J et al (2007) Analytical techniques in study of the environment fate of pharmaceuticals and personal-care products. Trends Anal Chem 26(6):557–568. https://doi.org/10.1016/j.trac.2006.11.004
Mokhtari SA, Farzadkia M, Gholami M (2017) Application of dispersive liquid–liquid microextraction as a simple assisted clean-up and preconcentration technique for GC/MS determination of selected PAHs extracted from sewage sludge by Soxhlet and ultrasound assisted extraction method. Desalin Water Treat 66(5):176–183. https://doi.org/10.5004/dwt.2017.20206
Topuz E, Sari S, Tas DO (2014) Optimization of diclofenac quantification from wastewater treatment plant sludge by ultrasonication assisted extraction. J Chromatogr B Anal Technol Biomed Life Sci 958(5):48–54. https://doi.org/10.1016/j.jchromb.2014.02.047
Azzouz A, Ballesteros E (2012) Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography–mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci Total Environ 419:208–215. https://doi.org/10.1016/j.scitotenv.2011.12.058
Viglino L, Prévost M, Sauvé S (2011) High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. Environ Monit 1(3):583–590. https://doi.org/10.1039/c0em00550a
Albero B, Sánchez-Brunete C, Miguel E (2013) Analysis of natural-occurring and synthetic sexual hormones in sludge Sánchez-Brunete-amended soils by matrix solid-phase dispersion and isotope dilution gas chromatography –tandem mass spectrometry. J Chromatogr A 1283:39–45. https://doi.org/10.1016/j.chroma.2013.01.113
Gineys N, Giroud B, Vulliet E (2010) Analytical method for the determination of trace levels of steroid hormones and corticosteroids in soil, based on PLE/SPE/LC–MS/MS. Anal Bioanal Chem 397(6):2295–2302. https://doi.org/10.1007/s00216-010-3787-4
Andreu V, Ferrer E, Rubio JL et al (2007) Quantitative determination of octylphenol, nonylphenol, alkylphenol ethoxylates and alcohol ethoxylates by pressurized liquid extraction and liquid chromatography–mass spectrometry in soils treated with sewage sludges. Sci Total Environ 378(1–2):124–129. https://doi.org/10.1016/j.scitotenv.2007.01.024
Wang YH, Wang QY, Hu LF et al (2015) Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Tai hu Lake in China. Environ Geochem Health 37(1):147–156. https://doi.org/10.1007/s10653-014-9637-0
Durán-Alvarez JC, Becerril-Bravo E, Castro VS (2009) The analysis of a group of acidic pharmaceuticals, carbamazepine, and potential endocrine disrupting compounds in waste water irrigated soils by gas chromatography–massspectrometry. Talanta 78(3):1159–1163. https://doi.org/10.1016/j.talanta.2009.01.035
Beck J, Totsche KU, Kögel-Knabner I (2008) A rapid and efficient determination of natural estrogens in soils by pressurised liquid extraction and gas chromatography–mass spectrometry. Chemosphere 71(5):954–960. https://doi.org/10.1016/j.chemosphere.2007.11.062
Anastassiades M, Lehotay SJ, Stajnbaher D (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. AOAC Int 86(2):412–431 (PMID:12723926)
Li YG, Chen ZL, Zhang R (2016) Simultaneous determination of 42 pesticides and herbicides in chicken eggs by UHPLC–MS/MS and GC–MS using a QuEChERS based procedure. Chromatographia 79:1165–1175. https://doi.org/10.1007/s10337-016-3132-y
Woźniak B, Kłopot A, Matraszek-Żuchowska I et al (2014) Determination of natural and synthetic oestrogens in surface water using gas chromatography-mass spectrometry. Bull Vet Inst Pulawy 58(4):603–611. https://doi.org/10.2478/bvip-2014-0093
Salvia MV, Vulliet E, Wiest L et al (2012) Development of a multi-residue method using acetonitrile-based extraction followed by liquid chromatography–tandem mass spectrometry for the analysis of steroids and veterinary and human drugs at trace levels in soil. J Chromatogr A 1245(6):122–133. https://doi.org/10.1016/j.chroma.2012.05.034
Fülöp I, Vari CE, Miklos A (2017) LC–MS/MS ESI Methods for the determination of oestrogens and androgens in biological matrix—a minireview. FARMACIA 65(4):485–493
Aufartováa J, Santanab CM, Rodríguezb JJS (2011) Determination of steroid hormones in biological and environmental samples using green microextraction techniques: an overview. Anal Chim Acta 704:33–46. https://doi.org/10.1016/j.aca.2011.07.030
Zhang K, Fent K (2018) Determination of two progestin metabolites (17α-hydroxypregnanolone and pregnanediol) and different classes of steroids (androgens, estrogens, corticosteroids, progestins) in rivers and wastewaters by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Sci Total Environ 610–611:1164–1172. https://doi.org/10.1016/j.scitotenv.2017.08.114
ICH (2005) Harmonised tripartite guideline, Q2B validation of analytical procedures: text and methodology, p 9
Konieczka P, Namieśnik J (2010) Estimating uncertainty in analytical procedures based on chromatographic techniques. J Chromatogr A 1217:882–889. https://doi.org/10.1016/j.chroma.2009.03.078
Asensio-Ramos M, Hernandez-Borges J, Ravelo-Perez LM et al (2010) Evaluation of a modified QuEChERS method for the extraction of pesticides from agricultural, ornamental and forestal soils. Anal Bioanal Chem 396(6):2307–2319. https://doi.org/10.1007/s00216-009-3440-2
Lillenberg M, Yurchenko S, Kipper K et al (2010) Presence of fluoroquinolones and sulfonamides in urban sewage sludge and their degradation as a result of composting. Int J Environ Sci Technol 7(2):307–312. https://doi.org/10.1007/BF03326140
Padilla-Sánchez JA, Plaza-Bolaños P, Romero-González R et al (2010) Application of a quick, easy, cheap, effective, rugged and safe-based method for the simultaneous extraction of chlorophenols, alkylphenols, nitrophenols and cresols in agricultural soils, analyzed by using gas chromatography–triple quadrupole-mass spectrometry/mass spectrometry. J Chromatogr A 1217:5724–5731. https://doi.org/10.1016/j.chroma.2010.07.004
AFNOR (NF EN 15662) (2009) Determination of pesticide residues using GC-MS and/or LC–MS/MS) following acetonitrile extraction/partitioning and clean-up by dispersive SPE—QuEChERS-method. France, p 11
AOAC Official Method 2007.01 (2007) Pesticides residues in food by acetonitrile extraction and partitioning with magnesium sulphate. AOAC Int 9
Han YT, Song L, Zou N et al (2016) Multi-residue determination of 171 pesticides in cowpea using modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J Chromatogr B 7(43):99–108. https://doi.org/10.1016/j.jchromb.2016.07.043
Charalampos KM, Stefanos T, Zisis V (2015) Determination of mycotoxins in pomegranate fruits and juices using a QuEChERS-based method. Food Chem 182:81–88. https://doi.org/10.1016/j.foodchem.2015.02.141
Nielsen KF, Ngemela AF, Jensen LB et al (2015) UHPLC–MS/MS determination of ochratoxin A and fumonisins in coffee using QuEChERS extraction combined with mixed-mode SPE purification. J Agric Food Chem 63(7):1029–1034. https://doi.org/10.1021/acs.jafc.5b00716
Bruzzoniti MC, Checchini L, De Carlo RM (2014) QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: a critical review. Anal Bioanal Chem 406(17):4089–4116. https://doi.org/10.1007/s00216-014-7798-4
Han Y, Song L, Zhao P (2016) Residue determination of glufosinate in plant origin foods using modified Quick Polar Pesticides (QuPPe) method and liquid chromatography coupled with tandem mass spectrometry. Food Chem 197(Part A):730–736. https://doi.org/10.1016/j.foodchem.2015.11.021
Kumirska J, Migowska N, Stepnowski P (2015) Simultaneous determination of non-steroidal anti-inflammatory drugs and oestrogenic hormones in environmental solid samples. Sci Total Environ 508:498–505. https://doi.org/10.1016/j.scitotenv.2014.12.020
Klinsunthorn N, Petsom A, Nhujak T (2011) Determination of steroids adulterated in liquid herbal medicines using QuEChERS sample preparation and high-performance liquid chromatography. Pharm Biomed Anal 55(5):1175–1178. https://doi.org/10.1016/j.jpba.2011.03.046
Gorga M, Insa S, Petrovic M (2014) Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci Total Environ 503–504:69–86. https://doi.org/10.1016/j.scitotenv.2014.06.037
Pérez RL, Escandar GM (2016) Multivariate calibration-assisted high-performance liquid chromatography with dual UV and fluorimetric detection for the analysis of natural and synthetic sex hormones in environmental waters and sediments. Environ Pollut 209:114–122. https://doi.org/10.1016/j.envpol.2015.11.024
Acknowledgements
The present work was funded by the Open Project of the Risk Assessment Lab for Agro-products (Beijing) Ministry of Agriculture (ZBZXKFKT201503), Open Project of the Risk Assessment Lab for Agro-products (Beijing) Ministry of Agriculture (ZBZXKFKT201701) and Special projects of construction of science and technology innovation ability of Beijing academy of agriculture and forestry sciences (KJCX20150408).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest with this study.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors. No compliance with ethical standards was involved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, S., Han, P., Li, A. et al. Simultaneous Determination of Trace Levels of 12 Steroid Hormones in Soil Using Modified QuEChERS Extraction Followed by Ultra Performance Liquid Chromatography–Tandem Mass Spectrometry (UPLC–MS/MS). Chromatographia 81, 435–445 (2018). https://doi.org/10.1007/s10337-017-3464-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3464-2