Abstract
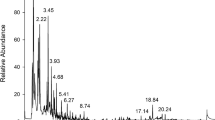
We developed and validated a simple and an accurate method for determination and quantification of glyphosate residue in vaccines. Vaccines were centrifuged in the first step of sample preparation to eliminate matrix effects. The sample was washed with a mixture of dichloromethane and chloroform, and was directly analysed without derivatisation. The extract was injected into a liquid chromatography tandem mass spectrometry (LC–MS/MS) system and liquid chromatography quadrupole-orbitrap mass spectrometry (LC-Q-Orbitrap/MS) with a Hypercarb column. The methods were validated through a series of assessments including specificity, limit of detection (LOD), limit of quantification (LOQ), linearity, precision and accuracy, recovery, and stability. In LC–MS/MS, LOD and LOQ of glyphosate in DPT and pneumococcal vaccines were 0.078 and 0.258 ng mL−1, whereas MMR was 0.156 and 0.515 ng mL−1. In Q-Orbitrap/MS, LOD and LOQ of glyphosate in DPT and pneumococcal vaccines were 2.425 and 8.803 ng mL−1, whereas MMR was 4.850 and 16.166 ng mL−1. The linear correlation coefficients (r 2) were higher than 0.999. Relative standard deviation (RSD) was 0.9–11.7% for both intra-day and inter-day precisions. Accuracy was evaluated to be in the range of 86.3–110.4% both for intra-day and for inter-day. Mean recoveries of three different fortification levels were within 93.1–108.3% for DPT and pneumococcal vaccines and 71.1–73.4% for MMR vaccine. The RSD of stability of spiked samples was within 8%.


Similar content being viewed by others
References
Dill GM, CaJacob CA, Padgette SR (2008) Glyphosate-resistant crops: adoption, use and future considerations. Pest Manag Sci 64:26–331
Tomlin CDS (2003) The pesticide Manual, 13th edn. British Crop Protection Council, Hampshire
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, vom Saal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. J Environ Health 15(19):1–13
Mesnage R, Defarge N, Spiroux de Vendômois J, Séralini GE (2015) Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol 84:133–153
Lee HL, Chen KW, Chi CH, Huang JJ, Tsai LM (2000) Clinical presentations and prognostic factors of a glyphosate—surfactant herbicide intoxication a review of 131 cases. Acad Emerg Med 7:906–910
Guyton KZ, Loomis D, Grosse Y, Ghissassi FE, Benbrahim-Talla L, Guha N, Scoccianti C, Mattock H, Straif K (2015) International Agency for Research on Cancer Monograph Working Group ILF. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol 16:490–491
Williams GM, Kroes R, Munro IC (2000) Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol 31:117–165
Gasnier C, Dumont C, Benachour N, Clair E, Chagnon MC, Eric G (2009) Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262:184–191
Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, Garrido T, Farré M (2012) Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402:2335–2345
Porterfield A(2016) Glyphosate herbicide in vaccines? Here is what concerned parents should know. GENETIC LITERACY PROJECT. USA. https://www.geneticliteracyproject.org/2016/09/20/glyphosateherbicide-vaccines-frightened-parents-know/. Accessed 20 Sept 2016
Moms Find Weed Killer in Childhood Vaccines: FDA and CDC MUST Test (2016) MOMS ACROSS AMERICA. USA. http://www.momsacrossamerica.com/glyphosate_in_vaccines_letter_to_fda_cdc_nih/. Accessed 10 Sept 2016
Fukata H, Miyagawa H, Yamazaki N, Mori C (2006) Comparison of Elisa- and LC–MS-based methodologies for the exposure assessment of bisphenol A. Toxicol Mech Methods 16:427–430
Granby K, Johannesen S, Vahl M (2003) Analysis of glyphosate residues in cereals using liquid chromatography–mass spectrometry (LC–MS/MS). Food Addit Contam 20:692–698
Jensena PK, Wujcika CE, McGuirec MK, McGuirec MA (2016) Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC–MS/MS. J Environ Sci Health Part B 51:254–259
Nagatomi Y, Yoshioka T, Yanagisawa M, Uyam A, Mochizuki N (2013) Simultaneous LC–MS/MS analysis of glyphosate, glufosinate, and their metabolic products in beer, barley tea, and their ingredients. Biosci Biotechnol Biochem 77:2218–2221
Yoshioka N, Asanob M, Kuseb A, Mitsuhashia T, Nagasakic Y, Uenob Y (2011) Rapid determination of glyphosate, glufosinate, bialaphos, and their major metabolites in serum by liquid chromatography–tandem mass spectrometry using hydrophilic interaction chromatography. J Chromatogr A 1218:3675–3680
Aris A, Leblanc S (2011) Maternal and fetal exposure to pesticides associated to genetically modified foods in eastern townships of Quebec, Canada. Reprod Toxicol 31:528–533
Arkan T, Molnár-Perl I (2015) The role of derivatization techniques in the analysis of glyphosate and aminomethyl-phosphonic acid by chromatography. Microchem J 121:99–106
Goscinny S, Unterluggauer H, Aldrian J, Hanot V, Masselter S (2012) Determination of glyphosate and its metabolite AMPA (aminomethylphosphonic acid) in cereals after derivatization by isotope dilution and UPLC-MS/MS”. Food Anal Methods 5:1177–1185
Teraza CPG, Dias CA, Aguiar MCS, Silvério FO, Fidêncio PH, Pinho GP (2014) A simple and efficient method for derivatization of glyphosate and AMPA using 9-fluorenylmethyl chloroformate and spectrophotometric analysis. J Braz Chem Soc 25:1194–1199
Botero-Coy AM, Ibanez M, Sancho JV, Hernandez F (2013) Direct liquid chromatography–tandem mass spectrometry determination of underivatized glyphosate in rice, maize and soybean. J Chromatogr A 1313:157–165
Li X, Xu J, Jiang Y, Chen L, Xu Y, Pan C (2009) Hydrophilic-interaction liquid chromatography (HILIC) with DAD and mass spectroscopic detection for direct analysis of glyphosate and glufosinate residues and for product quality control. Acta Chromatogr 21:559–576
(ICH) International conference on Harmonization (2005) Harmonized tripartite guideline, validation of analytical procedures: text and methodology Q2(R1). European Medicines Agency (EUMA), London
Acknowledgements
The authors would like to thank the Ministry of Food and Drug Safety (MFDSAAT2016) in Korea for the support of this study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Funding
This research was supported by Research Grants (MFDSAAT2016) from the Ministry of Food and Drug Safety (MFDS) in Korea.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.H., Park, H.N., Park, HJ. et al. Development and Validation of LC–MS/MS and LC-Q-Orbitrap/MS Methods for Determination of Glyphosate in Vaccines. Chromatographia 80, 1741–1747 (2017). https://doi.org/10.1007/s10337-017-3417-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3417-9