Abstract
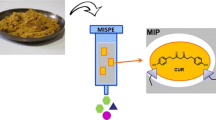
An efficient strategy of using molecularly imprinted solid-phase extraction and high-speed counter-current chromatography (HSCCC) were established for separation and purification of three analogues from Clematis akebioides for the first time. The three analogues, including 26.30 mg caffeic acid, 84.20 mg p-coumaric acid, and 44.40 mg ferulic acid, were obtained from 200.00 mg of the crude sample. The molecularly imprinted polymer was prepared with caffeic acid as template molecule and acrylamide as functional monomers. The results showed that the caffeic acid and its analogues could be well enriched. Then, the extract was purified by HSCCC using a two-phase solvent system consisting of chloroform/acetonitrile/water/acetic acid (10:5:4:0.0004, v/v) and a continuous sample introduction system was adopted. The three organic acids were identified by 1H-NMR and 13C-NMR.
Graphical abstract





Similar content being viewed by others
References
Wang WC, Li LQ (2005) A new system of classification of the genus Clematis (Ranunculaceae). J Syst Evol 43:431–488
Song CZ (2008) Study on chemical compositions of the Clematis montana and Clematis akebioides. Southwest Forestry University, Kunming, pp 1–42
Saija A, Tomaino A, Trombetta D et al (2000) In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm 199:39–47
Punithavathi VR, Prince PSM, Kumar R, Selvakumari J (2011) Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur J Pharmacol 650:465–471
Liobikas J, Majiene D, Trumbeckaite S et al (2011) Uncoupling and antioxidant effect of ursolic acid isolated rat heart mitochondria. J Nat Prod 74:1640–1644
Vari R, Archivio MD, Filesi C et al (2011) Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophage. J Nutr Biochem 22:409–417
Gong CC, Chen T, Liao ZX et al (2016) First separation of four aromatic acids and two analogues with similar structures and polarities from Clematis akebioides by high-speed counter-current chromatography. J Sep Sci 39:4660–4666
Yu YY, Zhang W, Cao SW (2007) Extraction of ferulic acid and caffeic acid with ionic liquids. Chin J Anal Chem 35:1726–1730
Peng LQ, Li Q, Chang YX et al (2016) Determination of natural phenols in olive fruits by chitosan assisted matrix solid-phase dispersion microextraction and ultrahigh performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1456:68–76
Peng XT, Li YN, Peng LJ, Feng YQ (2016) Rapid and sensitive detection of fipronil and its metabolites in edible oils by solid-phase extraction based on humic acid bonded silica combined with gas chromatography with electron capture detection. J Sep Sci 39:2196–2203
Chen FF, Wang GY, Shi YP (2011) Molecularly imprinted polymer microspheres for solid-phase extraction of protocatechuic acid in Rhizoma homalomenae. J Sep Sci 34:2602–2610
He XP, Tan LJ, Wang JT (2016) Determination of sulfadiazine in eggs using molecularly imprinted solid-phase extraction coupled with high-performance liquid. J Sep Sci 39:2204–2212
Yang X, Zhang ZH, Li JX et al (2014) Novel molecularly imprinted polymers with carbon nanotube as matrix for selective solid-phase extraction of emodin from kiwi fruit root. Food Chem 145:687–693
Miura C, Li H, Matsunaga H, Haginaka J (2015) Molecularly imprinted polymer for chlorogenic acid by modified precipitation polymerization and its application to extraction of chlorogenic acid from Eucommia ulmodies leaves. J Pharm Biomed 114:139–144
Saad EM, Madbouly A, Ayoub N et al (2015) Preparation and application of molecularly imprinted polymer for isolation of chicoric acid from Cichorium intybus L. Medicinal plant. Anal Chim Acta 877:80–89
Liu J, Li L, Tang H et al (2015) Preparation and characterization of erythromycin molecularly imprinted polymers based on distillation precipitation polymerization. J Sep Sci 38:3103–3109
Piletska EV, Burns R, Terry LA, Piletsky SA (2012) Application of a molecularly imprinted polymer for the extraction of kukoamine a from potato peels. J Agric Food Chem 60:95–96
Wang PL, Liu XM, Su XO, Zhu RH (2015) Sensitive detection of β-agonists in pork tissue with novel molecularly imprinted polymer extraction followed liquid chromatography coupled tandem mass spectrometry detection. Food Chem 184:72–79
Liu J, Zhang L et al (2015) Synthesis of metronidazole-imprinted molecularly imprinted polymers by distillation precipitation polymerization and their use as a solid-phase adsorbent and chromatography filler. J Sep Sci 38:1172–1178
Faure K, Bouju E, Suchet P, Berthod A (2013) Use of limonene in countercurrent chromatography: a green alkane substitute. Anal Chem 013(85):4644–4650
Liu YJ, Chen XF, Liu JX et al (2015) Three-phase solvent systems for the comprehensive separation of a wide variety of compounds from Dicranostigma leptopodum by high-speed counter-current chromatography. J Sep Sci 38:2038–2045
Qiu H, Xiao X, Li G (2012) Separation and purification of furanocoumarins from Toddalia asiatica (L.) Lam. using microwave-assisted extraction coupled with high-speed counter-current chromatography. J Sep Sci 35:901–906
Chen T, Liu YL, Zou DL et al (2014) Application of an efficient strategy based on liquid–liquid extraction, high-speed counter-current chromatography, and preparative HPLC for the rapid enrichment, separation, and purification of four anthraquinones from Rheum tanguticum. J Sep Sci 37:165–170
Shu XK, Duan WJ, Liu F et al (2014) Preparative separation of polyphenols from the flowers of Paeonia lactiflora Pall. by high-speed counter-current chromatography. J Chromatogr B 947–948:62–67
Xue K, Lue HT, Que BH et al (2010) High-speed counter-current chromatography preparative separation and purification of phloretin from apple tree bark. Sep Purif Technol 72:406–409
Wang X, Dong HJ, Liu YQ et al (2011) Application of high-speed counter-current chromatography for preparative separation of cyclic peptides from Vaccaria segetalis. J Chromatogr B 879:811–814
Tang H, Wu B, Chen K et al (2015) Separation of flavonoids from Millettia griffithii with high-performance counter-current chromatography guided by anti-inflammatory activity. J Sep Sci 38:523–529
Ruan C, Xiao XH, Li GK (2014) Microwave-assited extraction coupled with counter-current chromatography for the rapid preparation of flavonoids from Scutellaria barbata D. Don. J Sep Sci 37:1364–1369
Xu LL, Li AF et al (2010) Preparative isolation of neomangiferin and mangiferin from Rhizoma anemarrhenae by high-speed counter-current chromatography using ionic liquids as a two-phase solvent system modifier. J Sep Sci 33:31–36
Chen FF, Shi YP (2013) Application of molecularly imprinted solid phase extraction in the separation and determination of active constituents from natural compounds. Chin J Chromatogr 3:626–633
Dai X, Huang Q, Zhou B et al (2013) Preparative isolation and purification of seven main antioxidants from Eucommia ulmoides Oliv. (Du-zhong) leaves using HSCCC guided by DPPH-HPLC experiment. Food Chem 139:536–570
Bradow J, Riley F, Philippe L et al (2015) Automated solvent system screening for the preparative countercurrent chromatography of pharmaceutical discovery compounds. J Sep Sci 38:3983–3991
Zeng H, Liu Q, Yu J et al (2015) Separation of alpha-amylase inhibitors from Abelmoschus esculentus (L.). Moench by on-line two-dimensional high-speed counter-current chromatography target-guided by ultrafiltration-HPLC. J Sep Sci 38:3897–3904
Ito Y (2005) Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr A 1065:145–168
Jin X, Shi SM, Zhang DF, Zhu Z (2014) Chemical constituents of Andrographis paniculata (II). Chin Tradit Herb Drugs 45:164–169
Yang AM, Wu R, Yuan HJ et al (2015) Chemical constituents of Saxifraga umbellulata. Chem Nat Compd 51:330–331
Li GZ, Meng QY, Wang LJ et al (2015) Chemical constituents from Ziziphora clinopodioides. Chin Tradit Herb Drugs 46:2534–2539
Acknowledgements
This work was supported by the priority academic program development of Jiangsu higher Education Institutions (1107047002), Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (Guangxi Normal University) (CMEMR2016-B06 to ZXL), and Instrument Function Development Project of CAS (no. 2017g107).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Liu, H., Gong, C., Liao, Z. et al. Isolation and Purification of Three Analogues from Clematis akebioides by Molecularly Imprinted Solid-Phase Extraction and HSCCC. Chromatographia 80, 1651–1658 (2017). https://doi.org/10.1007/s10337-017-3406-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3406-z