Abstract
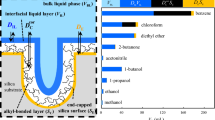
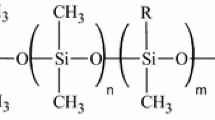
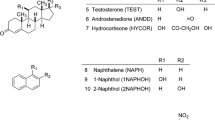
The experimentally known dependence in RP-HPLC of the retention factor k′ on octanol/water partition coefficient (K ow) has been examined based on solvophobic theory. The result showed that the dependence provides a means for the evaluation of phase ratio (Φ) of RP-HPLC columns, and of the equilibrium constant for a given compound and mobile phase. Using this theory, the phase ratio was evaluated for a set of seven different C18 columns (five having fully porous particles and two core–shell particles), and the equilibrium constants were calculated for four homologous series of compounds in two mobile phase systems. One mobile phase was methanol/aqueous solution of 0.1% H3PO4, and the other was acetonitrile/aqueous solution of 0.1% H3PO4. Besides providing the values for Φ for the evaluated columns, the results of the study indicated that for a specific composition of the mobile phase and for a given compound displaying only hydrophobic interactions, the equilibrium constant K(X) for different C-18 columns is basically the same. The data were further used to provide guidance in the selection of a chromatographic column for a specific separation based on K ow values and chemical structure of the analytes. The study indicated that the separation of compounds with identical polar groups (or no polar groups) and with very close values for the K ow cannot be achieved based only on hydrophobic interactions that dominate the separation on RP-type columns. Only column that displays polar interactions may provide a solution to such separations. For hydrocarbons with close K ow values, the separation cannot be achieved even on columns with some polarity. On the other hand, even compounds with equal K ow values, but with different functionalities can be separated on RP-HPLC columns without involving polar interactions. The compounds with different K ow values are expected to be easily separated on RP-HPLC columns.



Similar content being viewed by others
References
Vailaya A, Horváth C (1998) Retention in reversed-phase chromatography: partition or adsorption? J Chromatogr A 829(1–2):1–27
Dill KA (1987) The mechanism of solute retention in reversed-phase liquid chromatography. J Phys Chem 91(7):1980–1988
Moldoveanu SC, David V (2017) Selection of the HPLC method in chemical analysis. Elsevier, Amsterdam, p 126
Scott RPW, Kucera P (1975) Solute interactions with the mobile and stationary phases in liquid–solid chromatography. J Chromatogr A 112:425–442
Abraham MH, Ibrahim A, Zissimos AM (2004) Determination of sets of solute descriptors from chromatographic measurements. J Chromatogr A 1007(1–2):29–47
Rosés M, Bosch E (1993) Linear solvation energy relationship in reversed-phase liquid chromatography. Prediction of retention from a single solvent and a single solute parameter. Anal Chim Acta 274(1):147–162
Vitha M, Carr PW (2006) The chemical interpretation and practice of linear solvation energy relationships in chromatography. J Chromatogr A 1126(1–2):143–194
Tang B, Tian M, Lee YR, Row KH (2012) Using linear solvation energy relationship model to study the retention factor of solute in liquid chromatography. J Phys Org Chem 25(12):1058–1071
Sadek PC, Carr PW, Doherty RM, Kamlet MJ, Taft RW, Michael H, Abraham MH (1985) Study of retention processes in reversed-phase high-performance liquid chromatography by the use of the solvatochromic comparison method. Anal Chem 57(14):2971–2978
Carr PW, Doherty RM, Kamlet MJ, Taft RW, Melander W, Horvath C (1986) Study of temperature and mobile-phase effects in reversed-phase high-performance liquid chromatography by the use of the solvatochromic comparison method. Anal Chem 58(13):2674–2680
Torres-Lapasio JR, Garcıa-Alvarez-Coque MC, Roses M, Bosch E (2002) Prediction of the retention in reversed-phase liquid chromatography using solute–mobile phase–stationary phase polarity parameters. J Chromatogr A 955(1):19–34
Snyder LR, Maule A, Heebsch A, Cuellar R, Paulson S, Carrano J, Wrisley L, Chan CC, Pearson N, Dolan JW, Gilroy J (2004) A fast, convenient and rugged procedure for characterizing the selectivity of alkyl–silica columns. J Chromatogr A 1057(1–2):49–57
El Tayar N, van de Waterbeemd H, Testa B (1985) The prediction of substituent interactions in the lipophilicity of disubstituted benzenes using RP-HPLC. Quant Struct Act Relat 4(2):69–77
Moldoveanu S, David V (2015) Estimation of the phase ratio in reversed-phase high-performance liquid chromatography. J Chromatogr A 1381:194–201
Cheong WJ, Carr PW (1989) Limitations of all empirical single-parameter solvent strength scales in reversed-phase liquid chromatography. Anal Chem 61(14):1524–1529
Jandera P (1984) Reversed-phase liquid chromatography of homologous series. A general method for prediction of retention. J Chromatogr A 314:13–36
Jandera P (1993) Correlation of retention and selectivity of separation in reversed-phase high-performance liquid chromatography with interaction indices and with lipophilic and polar structural indices. J Chromatogr A 656(1–2):437–467
Jandera P, Colin H, Guiochon G (1982) Interaction indexes for prediction of retention in reversed-phase liquid chromatography. Anal Chem 54(3):435–441
Sinanoğlu O (1967) Intermolecular forces in liquids. In: Hirschfelder JO (ed) Advances in chemical physics, vol 12. Wiley, New York, pp 283–326
Horvath C, Melander W, Molnar I (1976) Solvophobic interactions in liquid chromatography with nonpolar stationary phases. J Chromatogr 125(1):129–156
Dorsey JG, Dill KA (1989) The molecular mechanism of retention in reversed-phase liquid chromatography. Chem Rev 89(2):331–346
Moldoveanu SC, David V (2013) Dependence of the distribution constant in liquid–liquid partition equilibria on the van der Waals molecular surface area. J Sep Sci 36(18):2963–2978
Liu TC (2010) Application of Kihara parameters in conventional molecular force fields. J Math Chem 48(2):363–369
Jasper JJ (1972) The surface tension of pure liquid compounds. J Phys Chem Ref Data 1(4):841–1009
Miller KJ, Savchik J (1979) A new empirical method to calculate average molecular polarizabilities. J Am Chem Soc 101(24):7206–7213
Moldoveanu SC, Savin A (1980) Aplicatii in chimie ale metodelor semiempirice de orbitali moleculari. Editura Academiei RSR, Bucuresti, pp 193–199
http://www.chemaxon.com. Accessed 7 Sept 2017
Ptitejean M (1994) On the analytical calculation of van der Waals surfaces and volumes: some numerical aspects. J Comput Chem 15(5):507–523
Talebian E, Talebian M (2013) A general review on the derivation of Clausius–Mossotti relation. Optik 124(16):2324–2326
Caiali E, David V, Aboul-Enein HY, Moldoveanu SC (2016) Evaluation of the phase ratio for three C18 high performance liquid chromatographic columns. J Chromatogr A 1435:85–91
Bondi A (1964) Van der Waals volumes and radii. J Phys Chem 68(3):441–451
Bastanov SS (2001) Van der Waals radii of elements. Inorg Mater 37(9):871–885
Rimmer CA, Simmons CR, Dorsey JG (2002) The measurement and meaning of void volumes in reversed-phase liquid chromatography. J Chromatogr A 965(1–2):219–232
https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface. Accessed 7 Sept 2017
Bocian S, Vajda P, Felinger A, Buszewski B (2009) Excess adsorption of commonly used organic solvents from water on nonend-capped C18-bonded phases in reversed-phase liquid chromatography. Anal Chem 81(15):6334–6346
Kazakevich YV, LoBrutto R, Chan F, Patel T (2001) Interpretation of the excess adsorption isotherms of organic eluent components on the surface of reversed-phase adsorbents. Effect on the analyte retention. J Chromatogr A 913(1–2):75–87
Moldoveanu SC, David V (2013) Essentials in modern HPLC separations. Elsevier, Amsterdam, pp 353–448
Connolly ML (1983) Analytical molecular surface calculation. J Appl Cryst 16(5):548–558
Acknowledgements
The authors are grateful to Mr. Ryan Zhang from Welch Materials Inc. for providing two of the studied columns (Boltimate C18 and Ultisil XB-C18).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Serban C. Moldoveanu declares that he has no conflict of interest. Author Edvin Caiali declares that he has no conflict of interest. Author Victor David declares that he has no conflict of interest.
Human/animal studies
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Moldoveanu, S.C., Caiali, E. & David, V. Phase Ratio and Equilibrium Constant in RP-HPLC Obtained from Octanol/Water Partition Constant Through Solvophobic Theory. Chromatographia 80, 1491–1500 (2017). https://doi.org/10.1007/s10337-017-3384-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3384-1