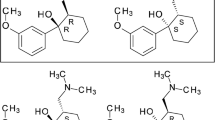
Abstract
Two separation techniques were developed for the determination of S-(−)darifenacin (DAR) in the presence of its R-(+) isomer: The first method is high performance liquid chromatography (HPLC) and the second is capillary electrophoresis (CE). Chiral separation for chromatographic HPLC method development was carried out for S-DAR on Daicel CROWNPAK CR (+) (5 μm, 4.0 × 150 mm) column which contains (3,3-diphenyl-1,1-binaphthyl)-crown-6 coated onto a 5.5 μm silica support. The mobile phase system was aqueous acidic 70 % HClO4 (pH 2.5): methanol in the proportion of 90:10 v/v. This current mobile phase was delivered at flow rate 0.8 mL min−1 using UV detector adjusted at 286 nm. In CE method, the enantiomers were separated using 50 μm inner diameter fused-silica capillary cut to total lengths of 31.2 cm using 50 mM phosphate buffer as background electrolyte adjusted to pH 2.5 by triethanolamine. A wide range of cyclodextrins (CDs) were used such as highly sulfated α, γ CDs, hydroxyl propyl-β-CD and sulfobutyl ether-β-CD as chiral selectors. The effects of chiral additives regarding its concentration and content of organic modifier on the enantioseparation were investigated. Linear concentration ranges were from 2.5 to 50 and 40 to 300 μg mL−1 with detection limits 0.67 and 12.28 μg mL−1 for chromatographic HPLC and electrophoretic CE methods, respectively. The two methods were validated according to ICH guidelines with respect to linearity, accuracy, precision, LOQ, LOD and robustness. The suggested methods are suitable for separation and quantitation of S-DAR in tablets.







Similar content being viewed by others
References
O'Neil MJ (2013) The merck index : an encyclopedia of chemicals, drugs, and biologicals, 15th edn. Royal Society of Chemistry, London
Haab F, Stewart L, Dwyer P (2004) Eur Urol 45:420–429
Haab F (2005) Women’s health 1:331–343. doi:10.2217/17455057.1.3.331
Steers WD (2006) Urol Clin N Am 33:475–482
Kerbusch T, Milligan PA, Karlsson MO (2004) Br J Clin Pharmacol 57:170–180
Skerjanec A (2006) Clin Pharmacokinet 45:325–350
Ozoemena KI, Stefan RI, van Staden JF, Aboul-Enein HY (2004) Talanta 62:681–685
Nguyen LA, He H, Pham-Huy C (2006) Int J Biomed Sci 2:35–100
Gubitz G, Schmid MG (2006) Mol Biotechnol 32:159–180
Beesley TE, Scott RW (1998) Chiral chromatography. Wiley, New York
Merola G, Fu H, Tagliaro F, Macchia T, McCord BR (2014) Electrophoresis 35:3231–3241
Radhakrishnanand P, Subba DV, Himabindu V (2008) Chromatographia 68:1059–1062
Venn RF, Goody RJ (1999) Chromatographia 50:407–414
Kaye B, Herron WJ, Macrae PV, Robinson S, Stopher DA, Venn RF, Wild W (1996) Anal Chem 68:1658–1660
Nazeerunnisa M, Garikapati L, Bethanabhatla S (2014) Am J Anal Chem 5:239–1248
Krishna C, Kumar SS, ThirveLl S (2012) Int J Pharmacy and Pharmaceutical Sci 5:346–352
Murthy MV, Krishnaiah C, Srinivas K, Rao KS, Kumar NR, Mukkanti K (2013) J Pharm Biomed Anal 72:40–50
Hefnawy MM, Alanazi AM, Abounassif MA, Mohammed MS, Attia SM, Mostafa GA (2014) Talanta 121:37–42
Jeon SH, Kim M, Han H-K, Lee W (2010) J Arch Pharm Res 33:1419–1420
Toğrul M, Demirel N, Kaynak B, Özbey S, Hoşgören H (2005) J Incl Phenom Macrocycl Chem 50:165–171
Lee W, Baek CS, Lee K (2002) Bull Korean Chem Soc 23:1677–1679
Hiraoka M (1997) Crown ethers and analogous compounds. Elsevier, Amsterdam
Ariga K, Kunitake T (2006) Supramolecular chemistry-fundamentals and applications: advanced textbook. Springer, Berlin
Lee W, Jin JY, Baek CS (2005) Microchem J 80:213–217
Williams AB, Vigh G (1997) J Chromatogr A 777:295–309
Valkó IE, Billiet HAH, Frank J, Luyben KCAM (1994) Chromatographia 38:730–736
Tanaka Y, Terabe S (1997) J Chromatogr A 781:151–160
Chankvetazede B (1997) Capillary electrophoresis in chiral analysis. Wiely, England
ICH Harmonized Tripartie Guidline Q2B R1 (2005) Validation of analytical procedures: text and methodology. http://www.ich.org
Acknowledgments
The authors would like to thank Institute of Scientific Research and Revival of Islamic Heritage at Umm AlQura University (project ID 43510001) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bawazeer, S.S., Abdel-Megied, A.M. & Bebawy, L.I. Enantiospecific HPLC and CE Methods for Separation and Determination of S-Darifenacin in Pharmaceutical Formulations. Chromatographia 79, 1533–1542 (2016). https://doi.org/10.1007/s10337-016-3171-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3171-4