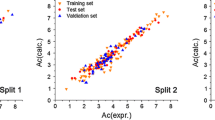
Abstract
A quantitative structure–retention relationship (QSRR) study was performed to correlate descriptors representing molecular structures to the Kováts retention indices of polycyclic aromatic hydrocarbon compounds. The complete set of 37 compounds was divided randomly into a training set of 26 compounds and a test set of 11 compounds. In the pool of descriptors calculated using Dragon and Hyperchem software, the modified Harary index (\( H^{\prime\prime} \)) developed by our team was added. The best selected descriptors by genetic algorithm to build the three linear models were: the first-order Randić connectivity index (\( {}^{1}\chi \)), \( H^{\prime\prime} \) and the third-order valence connectivity index (\( {}^{3}\chi_{p}^{v} \)). The selected model was compared with the one built with \( {}^{1}\chi \), the first Harary index (implanted in Dragon H) and \( {}^{3}\chi_{p}^{v} \). Statistical analysis showed that the model with modified Harary index (R 2 = 99.25, SD 5.82, F = 1517.74) produces better correlation with Kováts retention indices than the one implanted in Dragon (R 2 = 98.20, SD 9.02, F = 626.09). The final QSRR was internally and externally validated. The leave-one-out, cross-validation, bootstrapping, and y-randomization test indicated that the final model is robust and has no chance correlation. The external validations indicated that the model with modified Harary index showed a good predictive power. The mechanistic interpretation of QSRR model was carried out according to the definition of descriptors. Therefore, the result meets the five principles recommended by the Organization for Economic Co-operation and Development for validation of QSRR model.






Similar content being viewed by others
References
Karelson M, Lobanov VS, Katritzky AR (1996) Quantum-chemical descriptors in QSAR/QSPR studies. Chem Rev 96:1027–1044
Santos FJ, Galceran MT (2002) The application of gas chromatography to environmental analysis, TrAC. Trends Anal Chem 21:672–685
Kaliszan R, Foks H (1977) The relationship between the RM values and the connectivity indices for pyrazine carbothioamide derivatives. Chromatographia 10:346–349
Kaliszan R (1977) Correlation between the retention indices and the connectivity indices of alcohols and methyl esters with complex cyclic structure. Chromatographia 10:529–531
Michotte Z, Massart DL (1977) Molecular connectivity and retention indexes. J Pham Sci 66:1630–1632
Héberger K (2007) Quantitative structure-(chromatographic) retention relationships. J Chromatogr A 1158:273–305
Ivanciuc O, Ivanciuc T, Balaban AT (1998) Quantitative structure-property relationship study of normal boiling points for halogen-/oxygen-/sulfur-containing organic compounds using the CODESSA program. Tetrahedron 54:9129–9142
Pompe M, Novic M (1999) Prediction of gas chromatographic retention indices using topological descriptors. J Chem Inf Comput Sci 39:59–67
Junkes BS, Amboni RDMC, Yunes RA, Heinzen VEF (2003) Prediction of the chromatographic retention of saturated alcohols on stationary phases of different polarity applying the novel semi-empirical topological index. Anal Chim Acta 477:29–39
Lu C, Guo W, Yin C (2006) Quantitative structure-retention relationship study of the gas chromatographic retention indices of saturated esters on different stationary phases using novel topological indices. Anal Chim Acta 561:96–102
Payares P, Diaz D, Olivero J, Vivas R, Gomez I (1997) Prediction of the gas chromatographic relative retention times of flavonoids from molecular structure. J Chromatogr A 771:213–219
Tulasamma P, Reddy KS (2006) Quantitative structure and retention relationships for gas chromatographic data: application to alkyl pyridines on apolar and polar phases. J Mol Graph Model 25:507–513
Randić M (1997) On characterization of chemical structure. J Chem Inf Comput Sci 37:672–687
Kier L, Hall L (2002) The meaning of molecular connectivity: a bimolecular accessibility model. Croat Chem Acta 75:371–382
Drosos JC, Rhenals MV, Reyes RV (2010) Quantitative structure-retention relationships of polycyclic aromatic hydrocarbons gas-chromatographic retention indices. J Chromatogr A 1217:4411–4421
Ghavami R, Faham S (2010) QSRR Models for Kováts’ retention indices of a variety of volatile organic compounds on Polar and apolar GC stationary phases using molecular connectivity indexes. Chromatographia 72:893–903
Polyakova Y, Long MJ, Kyung HR (2006) Linear regression based QSPR models for the prediction of the retention mechanism of some nitrogen containing heterocycles. J Liq Chromatogr Rel Technol 29:533–552
Mihalić Z, Trinajstić N (1992) A graph-theoretical approach to structure-property relationships. J Chem Educ 69:701–712
Wiener H (1947) Structural determination of paraffin boiling points. J Am Chem Soc 69:17–20
Ivanciuc O, Balaban TS, Balaban AT (1993) Design of topological indices. Part 4. Reciprocal distance matrix, related local vertex invariants and topological indices. J Math Chem 12:309–318
Plavšić D, Nikolić S, Trinajstić N, Mihalić Z (1993) On the Harary index for the characterization of chemical graphs. J Math Chem 12:235–250
Lučić B, Miličević A, Nikolić S, Trinajstić N (2002) Harary Index—twelve years later. Croat Chem Acta 75:847–868
Zhou B, Cai X, Trinajstić N (2008) On Harary index. J Math Chem 44:611–618
XX Li, YZ Fan (2012) The connectivity and the Harary Index of a graph. arXiv:1207.2393 [math.CO]
Todeschini R, Consonni V (2000) Handbook of molecular descriptors. Wiley, Weinheim, p 70, 6
Dragon 5.5 (2009) TALETE. http://www.talete.mi.it
Lee M, Vassilaros D, White C, Novotny M (1979) Retention indices for programmed-temperature capillary-column gas chromatography of polycyclic aromatic hydrocarbons. Anal Chem 51:768–773
Marvin 5.9.0 (2012) Chem Axon. http://www.chemaxon.com
Hyperchem 6.02 (2000) Hypercube. http://www.hyper.com
SIMCA-P 10.0.4 (2003) Umetrics AB, Umeå. http://www.umetrics.com
MobyDigs 1.1 (2009) TALETE. http://www.talete.mi.it
Abdi H (2003) Partial least squares (PLS) regression; multivariate analysis. In: Lewis-Beck M (ed) Encyclopedia of social sciences research methods. Sage, pp 792–795. http://www.utdallas.edu/~herve/Abdi-PLS-pretty.pdf
Tobias RD (1997) An introduction to partial least squares regression. SAS Institute, Cary. http://www.ats.ucla.edu/stat/sas/library/pls.pdf
Eriksson L, Hermens J, Johansson E, Verhaar H, Wold S (1995) Multivariate analysis of aquatic toxicity data with PLS. Aquat Sci 57:217–241
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Mod 20:269–276
Ojha PK, Mitra I, Das RN, Roy K (2011) Further exploring r2 m metrics for validation of QSPR models. Chemom Intell Lab Syst 107:194–205
Randić M (1975) On characterization of molecular branching. J Am Chem Soc 97:6609–6615
Kier LB, Hall LH (1981) Derivation and significance of valence molecular connectivity. J Pharm Sci 70:583–589
(2004) OECD principles for the validation of (Q)SARs, adopted by 37th Joint Meeting of Chemicals Committee and Working Party on chemicals, pesticides and biotechnology; Paris 17–19. https://eurl-ecvam.jrc.ec.europa.eu/laboratories-research/predictive_toxicology/background/oecd-principles
Tenenhaus M, La régression PLS (1998) Théorie et pratique, Paris Éditions Technip
Wold S (1995) PLS for multivariate linear modeling. In: van de Waterbeemd H (ed) QSAR: chemometric methods in molecular design, methods and principles in medicinal chemistry, 2nd edn. Verlag Chemie, Weinheim, pp 195–218
Wold S, William J (1983) Dunn III multivariate quantitative structure-activity relationships (QSAR): conditions for their applicability. J Chem Inf Comput Sci 23:6–13
Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S (2006) Multi and megavariate data analysis Part I: basic principles and applications, 2nd edn. Umetrics Academy, Umea
Acknowledgments
The authors thank the general directorate for scientific research and technological development of the Algerian ministry of high education and scientific research for supporting this work and Kim Koffolt for her help to improve the write-up quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the general directorate for scientific research and technological development of the Algerian ministry of high education and scientific research.
Conflict of interest
All authors declared that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Touhami, I., Haddag, H., Didi, M. et al. Contribution of Modified Harary Index to Predict Kováts Retention Indices for a Set of PAHs. Chromatographia 79, 1023–1032 (2016). https://doi.org/10.1007/s10337-016-3120-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3120-2