Abstract
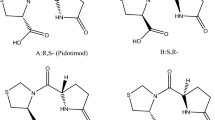
A new, simple, selective, and robust normal-phase method for the accurate quantification of all the four stereoisomers and one geometrical isomer of pitavastatin calcium (PIT) in drug substances and drug products was developed. The method is capable of quantifying all the isomers in the presence of other related substances. Separation was achieved using immobilized amylose stationary phase (Chiralpak IA) with a mixture of n-heptane, 1-butanol, methanol, formic acid, and diethylamine. Multivariate analysis and statistical tools were used to develop this highly robust method in a short span of time. A central composite design was employed to study the main effects and interactions of the independent variables. The method exhibited consistent, high-quality recoveries [97.3 ± 1.7 to 99.3 ± 2.1 (mean ± RSD)] with a high precision for all the isomers. Linear regression analysis revealed an excellent correlation between peak responses and concentrations (r 2 values of 0.9990–0.9998) for the isomers. The method is sensitive enough to quantify any isomers above 0.02 % and detect any isomer above 0.006 % in PIT. Forced degradation studies proved that the method is specific for isomers. m/z values were determined for the major degradants and their possible structures were proposed on the basis of the known reactivity.





Similar content being viewed by others
References
Stender S, Budinski D, Hounslow N (2013) Pitavastatin shows greater lipid lowering efficacy over 12 weeks than pravastatin in elderly patients with primary hypercholesterolemia or combined dyslipidaemia. Eur J Prev Cardiol 20:29–39
Stender S, Budinski D, Hounslow N (2013) Pitavastatin demonstrates long-term efficacy, safety and tolerability in elderly patients with primary hypercholesterolaemia or combined (mixed) dyslipidaemia. Eur J Prev Cardiol 20:40–53
Gumprecht J, Gosho M, Budinski D, Hounslow N (2011) Comparative long-term efficacy and tolerability of pitavastatin 4 mg and atorvastatin 20–40 mg in patients with type 2 diabetes mellitus and combined (mixed) dyslipidaemia. Diabetes Obes Metab 13:1047–1055
Eriksson M, Budinski D, Hounslow N (2011) Long-term efficacy of pitavastatin versus simvastatin. Adv Ther 28:799–810
Ose L, Budinski D, Hounslow N, Arneson V (2010) Long-term treatment with pitavastatin is effective and well tolerated by patients with primary hypercholesterolemia or combined dyslipidemia. Atherosclerosis 210:202–208
FDA/Center for Drug Evaluation and Research (2009) Label Information—drugs at FDA, MD-20993. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022363s010lbl.pdf. Accessed Oct 2012
Patrizia G, Maria CP, Giuseppina G, Anna MM, Elena C, Simona P, Antonietta S, Chiara L, Maurizio B (2012) Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev 64:102–146
Pharma Book Syndicate (2009) Hyderabad, India. http://www.pharmabooksyndicate.com/issues/309.pdf. Accessed Mar 2009
Ramakrishna N, Koteshwara M, Vishwottam K (2007) Chromatography-mass spectrometry methods for the quantification of statins in biological samples. J Pharm Biomed Anal 44:379–387
Jianzhong ST, Xiao X, Jian L, Xingjiang H, Junchun C, Lihua W, Mingzhu H, Huili Z (2009) Determination of pitavastatin in human plasma by LC–MS–MS. Chromatographia 69:1041–1047
Antony RG, Pannala RR, Nimmakayala S, Jadi S (2010) Degradation pathway for pitavastatin calcium by validated stability indicating UPLC method. Am J Anal Chem 1:83–90
Satheesh NK, Baghyalakshmi J (2007) Determination and quantification of pitavastatin calcium in tablet dosage formulation by HPTLC method. Anal Lett 40:2625–2632
Hiral JP, Bhanubhai NS, Natubhai JP, Bhavesh HP (2008) A simple and sensitive HPTLC method for quantitative analysis of pitavastatin calcium in tablets. J Planar Chromatogr 21:267–270
Cheng X, Wang L, Yang G, Cheng J, Zhang Y (2010) Chiral separation of pitavastatin calcium enantiomers by capillary zone electrophoresis. Se Pu 28:1089–1093
Vishnu MM, Krishnaiah C, Kodithyala J, Katkam S, Mukkanti K, Ramesh K, Gautam S (2011) Enantioseparation of palonosetron hydrochloride and its related enantiomeric impurities by computer simulation and validation. Am J Anal Chem 2:437–446
ICH (2012) Development and manufacture of drug substances Q11. ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf. Accessed 1 May 2012
LCGC (2008) A strategy for developing HPLC methods for chiral drugs. http://www.chromatographyonline.com/lcgc/data/articlestandard/lcgc/122008/504160/article.pdf. Accessed Feb 2008
Al-Othman Z, Ali I, Asim M, Khan TA (2012) Recent trends in chiral separations on immobilized polysaccharides CSPs. Comb Chem High Throughput Screen 15(4):339–346
Irving WW, Rose MS (1987) Resolution of enantiomeric aromatic alcohols on a cellulose tribenzoate high-performance liquid chromatography chiral stationary phase: a proposed chiral recognition mechanism. J Chromatogr A 411:139–151
Brian LH (2010) In: Alain B (ed) Chiral recognition in separation methods: mechanisms and applications. Springer, New York
Chiral Technologies Europe (2004) Instruction manual for Chiralpak-IA column. http://www.mz-at.de/pdf/Chiralpak_IA_Manual.pdf. Accessed April 2004
Paul F, Mark H, Jennifer Y (2012) In: Wei Z, Berkeley C (eds) Green techniques for organic synthesis and medicinal chemistry. Wiley, West Sussex
Zhang T, Franco P, Nguyen D, Hamasaki R, Miyamoto S, Ohnishi A, Murakami T (2012) Complementary enantiorecognition patterns and specific method optimization aspects on immobilized polysaccharide-derived chiral stationary phases. J Chromatogr A 1269:178–188
Toussaint B, Duchateau AL, Vanderwal S, Albert A, Hubert P, Crommen J (2000) Determination of the enantiomers of 3-tert-butylamino-1,2-propanediol by high-performance liquid chromatography coupled to evaporative light scattering detection. J Chromatogr A 890:239–249
Tang Y (1996) Significance of mobile phase composition in enantioseparation of chiral drugs by HPLC on a cellulose-based chiral stationary phase. Chirality 8:136–142
Parajo JC, Alonso JL, Lage MA, Vazquez D (1992) Empirical modeling of eucalyptus wood processing. Bioprocess Eng 8:129–136
Suresh KR, Hariram B, Divya G, Srinivasu MK, Srinivas K, Sagyam RR (2012) Development of a RP-LC method for a diastereomeric drug valganciclovir hydrochloride by enhanced approach. J Pharm Biomed Anal 70:101–110
Grobelny P, Viola G, Vedaldi D, Dallacqua F, Gliszczynska S A, Mielcarek J (2009) Photo stability of pitavastatin—a novel HMG-CoA reductase inhibitor. J Pharm Biomed Anal 50:597–601
ICH (2005) Validation of analytical procedures: text and methodology Q2 (R1). ICH harmonised tripartite guideline. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. Accessed Nov 2005
Acknowledgments
The authors wish to thank Dr. L. Kalyanaraman, Dr. Vyas, and the management of Dr. Reddy’s Laboratories for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hariram, B., Kumar, R.S., Jayashree, A. et al. Development of Stereoselective Method for the Quantification of Stereoisomers and Geometrical Isomer of Pitavastatin Calcium by Enhanced Approach. Chromatographia 77, 901–912 (2014). https://doi.org/10.1007/s10337-014-2693-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2693-x