Abstract
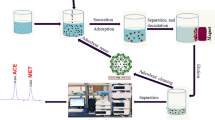
Aqueous two-phase flotation (ATPF) is especially applicable to bioseparation. However, there is no previous work reporting on its application in enantioseparation. Using phenylsuccinic acid (H2A) as the model enantiomers, ATPF was first introduced into the field of chiral separation. The contributions of a series of ATPF systems to the enantioseparation were investigated. The results indicated that an appropriate increase in the amount of phase-forming components and a decrease in pH values (from 5.5 to 2) are both beneficial for the enantioseparation. Enantioselective flotation and partitioning behavior are mainly dependent on pH values of the solutions, types, and concentrations of chiral selectors. Furthermore, salt, PEG, flow rates of air, and flotation time also have some effects on the enantioseparation. Under the optimal conditions, the enantioselectivity was calculated in terms of the separation factor (α) and enantiomer excess (e.e. %) as 1.99 and 23.49 %, respectively. Finally, the most reasonable mechanisms for H2A enantioseparation in ATPF system have been proposed. An ATPF system composed of PEG2000/(NH4)2SO4 was proved to have the best ability for the separation of H2A enantiomers. The explorations in our study will further enrich the enantioseparation methods and pave the way for the application of ATPF in the enantioseparation field.






Similar content being viewed by others
References
Horvath JD, Koritnik A, Kamakoti P, Sholl DS, Gellman AJ (2004) J Am Chem Soc 126:14988–14994
Maier NM, Franco P, Lindner W (2001) J Chromatogr A 906:3–33
Lee NH, Frank CW (2002) Polymer 43:6255–6262
Ginesta X, Pericas MA, Riera A (2002) Tetrahedron Lett 43:779–782
Tong S, Yan J, Guan YX, Lu Y (2011) J Chromatogr A 1218:5602–5608
Tang KW, Song LT, Liu YB, Pan Y, Jiang XY (2010) Chem Eng J 158:411–417
Schuur B, Winkelman JGM, de Vries JG, Heeres HJ (2010) Chem Eng Sci 65:4682–4690
Zhang P, Cai J, Tang K, Liu Y, Liu Y, Yan J (2012) Chem Eng Process 61:16–22
Tang K, Luo J, Zhang P, Zhou C, Yi J (2013) Chem Eng Res Des 91:919–924
Bi PY, Dong HR, Yuan YC (2010) Sep Purif Technol 75:402–406
Bi PY, Li DQ, Dong HR (2009) Sep Purif Technol 69:205–209
Li M, Dong HR (2010) Sep Purif Technol 73:208–212
Pakhale SV, Vetal MD, Rathod VK (2013) Sep Sci Technol 48:984–989
Chen X, Yang W, Jiang X, Jiao F, Tian L (2012) Tetrahedron Asymmetry 23:1227–1233
Wang LJ, Hu SQ, Guo QL, Yang GL, Chen XG (2011) J Chromatogr A 1218:1300–1309
Tan L, Long Y, Jiao F, Chen X (2011) J Iran Chem Soc 8:889–896
Tang KW, Miao JB, Zhou T, Liu YB (2011) Chinese J Chem Eng 19:397–403
Xing JM, Li FF (2012) J Chem Technol Biotechnol 87:346–350
Varga G, Fodor G, Ilisz I, Szemán J, Visy J, Szente L, Péter A (2012) J Pharm Biomed 70:71–76
Man RL, Wang ZH, Tang KW (2009) J Cent South Univ Technol 16:201–205
Chen XQ, Dong QL, Yu JQ, Jiao FP (2013) J Chem Technol Biotechnol 88:1545–1550
Tan ZJ, Li FF, Xu XL (2012) Bioprocess Biosyst Eng 36:1105–1113
Zafarani-Moattar MT, Hamzehzadeh S (2005) Calphad 29:1–6
Bi PY, Dong HR, Dong J (2010) J Chromatogr A 1217:2716–2725
Bayati F, Shayegan J, Noorjahan A (2011) J Pet Sci Eng 80:26–31
Dong HR, Bi PY, Wang SH (2005) Anal Lett 38:257–270
Acknowledgments
We would like to acknowledge the financial support from the China National Natural Science Foundation (No. 21276282) and Hunan National Natural Science Foundation (No. 14JJ2014).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhuang, J., Yang, W., Chen, X. et al. Enantioseparation of Phenylsuccinic Acid Enantiomers Using Aqueous Two-Phase Flotation and Their Determination by HPLC and UV Detection. Chromatographia 77, 679–685 (2014). https://doi.org/10.1007/s10337-014-2668-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-014-2668-y