Abstract
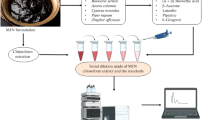
Hydrastis canadensis L. is one of the important herbal medicinal plants, which is used for homeopathic preparations. It contains berberine, hydrastine as major and canadine, hydrastinine, berberastine and canadaline as minor bioactive compounds. The aim was to develop (I) a sensitive mass spectrometric-based quantitative/qualitative method for berberine in homeopathic dilutions of H. canadensis, (II) with identification of berberine and its structurally related compound present in the homeopathic preparation of H. canadensis using predictive MRM workflow, (III) confirmatory analysis for the structurally related compound in homeopathic dilutions of H. canadensis using high-resolution mass spectrometer. Different mass spectrometric-based workflows (MRM to EPI; pMRM to EPI and IDA-DBS) were used to develop the method to analyze the homeopathic dilutions of H. canadensis. A high-throughput liquid chromatography-tandem mass spectrometry method using MRM to EPI workflow was developed to analyze berberine in homeopathic preparation of H. canadensis. Multiple reaction monitoring transitions of 336.0/292.08 and 336.0/277.9 were selected for developing the quantitation method of berberine. The method was linear from 5.0 to 2000.0 pg/mL. The validated method has been successfully applied for the analysis of different potencies of homeopathic preparation of H. canadensis. Predictive MRM to enhanced product ion workflows was used to identify and confirm the structurally related molecules of berberine. Non targeted information dependent acquisition-DBS method was developed to identify the compounds in H. canadensis mother tincture using high-resolution mass spectrometry. The established method using different mass spectrometric workflows facilitates the convenient and fast quality control analysis for the herbal preparation of H. canadensis.









Similar content being viewed by others
References
Weber HA, Zart MK, Fergusson SL, Greaves JG, Clark AP, Harris RK, Overstreet D, Smith C (2001) J Liq Chrom Rel Technol 24(1):87–95
Weber HA, Zart MK, Molloy HM, Brien BMO, Moody LA, Clark AP, Harris RK, Overstreet D, Smith C (2003) J Agric Food Chem 51:7352–7360
Blumental M (1999) Herbalgram 47:64–65
United States Pharmacopeial Convention: USP 26-NF21 Supplement 1 2003
Rojsanga P, Gritsanapan W, Suntornsuk L (2006) Med Princ Pract 15:373–378
Chen YR, Wen KC, Her GR (2000) J Chromatogr A 866:273–280
Suto K, Kakinuma S, Ito Y, Sagara K, Iwasaki H, Itokawa H (1997) J Chromatogr A 786:371–376
Chattopadhyay SK, Kumar S (2007) Biomed Chromatogr 21:55–66
Zhang Y, Wu W, Han F, Chen Y (2008) Biomed Chromatogr 22:1360–1367
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH (2007) J Chromatogr A 853:183–189
Gratz SR, Flurer CL, Wolnik KA (2004) J Pharm Biomed Anal 36:525–533
Lee MC, Tsao CH, Iou SC, Chuang WC, Sheu SJ (2003) J Sep Sci 26:818–822
Hua W, Ding L, Chen Y, Gong B, He J, Xu G (2007) J Pharm Biomed Anal 44:931–937
Lu T, Liang Y, Song J, Deng Y, Xie L, Wang J, Liu X (2006) J Pharm Biomed Anal 40:1218–1222
Deng Y, Liao Q, Li S, Bi K, Pan B, Xie Z (2008) J Chromatogr B Analyt Technol Biomed Life Sci 863:195–205
Zuo F, Zhou ZM, Zhang Q, Mao D, Xiong YL, Wang YL, Yan MZ, Liu ML (2003) Biol Pharm Bull 26:911–915
Guidance for Industry: Bioanalytical method validation (2001) U.S. Department of Health and Human services food and drug administration center for drug evaluation and research (CDER) center of veterinary medicine (CVM)
Brown PN, Paley LA, Roman MC, Chan M (2008) Pharma Biol 46:135–139
Brown PN, Roman MC (2008) J AOAC Int 91:694–701
Luo X, Chen B, Yao S (2005) Talanta 66:103–106
Hopfgartner G, Varesio E, Tschappat V, Grivet C, Bourgogne E, Leuthold LA (2004) J Mass Spectrom 39:845–855
ICH Q2B (1996) Validation of analytical procedure: methodology, international conference on harmonization of technical requirements for registration of pharmaceuticals for human use
Gupta PK, Hubbard M, Gurley B, Hendrickson HP (2009) J Pharm Biomed Anal 49:1021–1026
Zuo F, Nakamura N, Akao T, Hattori M (2006) Drug Metab Dispos 34:2064–2072
Carmai S (2010) ABSCIEX™ Tech note 1
Liu HF, Jones E (2008) AB SCIEX™ Tech note1
Tims MC (2006) Digital Repository at the University of Maryland. http://drum.lib.umd.edu/handle/1903/4052
Guillarme D, Schappler J, Rudaz S, Lveuthey J (2010) Trends Anal Chem 29:15–27
Shawn MR, Hsiao S, Foo C (2006) J Chromatogr B 836:1–14
Mueller CA, Weinmann W, Dresen S, Schreiber A, Gergov M (2005) Rapid Commun Mass Spectrum 19:1332–1338
Yves Le Blanc JC, Bloomfiled N (2004) Presented in ASMS, TPM, p 224
Bradley P (1992) British herbal compendium vol. 1. Bournemouth, UK: BHMA
Galeffi C, Cometa MF, Tomassini L, Nicoletti M (1997) Planta Med 63:194–195
Govindan M, Govindan G (2000) Fitoterapia 71:232–235
Upton R (2001) American Herbal Pharmacopoeia
Hwang BY, Roberts SK, Chadwick LR, Wu CD, Kinghorn AD (2003) Planta Med 69:623–627
Wang CZ, Kim KE, Du GJ, Qi LW, Wen XD, Li P, Bauer BA, Bissonnette M, Musch MW, Chang EB, Yuan CS (2011) Am J Chin Med 39:1161–1171
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pillai, M.G., Kumar, A., Sharma, R. et al. LC–MS Based Workflows for Qualitative and Quantitative Analysis for Homeopathic Preparation of Hydrastis canadensis . Chromatographia 77, 119–131 (2014). https://doi.org/10.1007/s10337-013-2577-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2577-5