Abstract
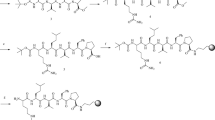
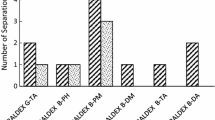
Two enantiomerically pure amines, viz., (R)-(+)-naphthylethyl amine and (S)-(+)-1-benzyl-3-aminopyrrolidine, were used as chiral auxiliaries for nucleophilic substitution of chlorine atoms in cyanuric chloride or its 6-butoxy derivative. The chiral derivatizing reagents so obtained were characterized and their chiral purity was ascertained. Diastereomers of 15 dl-proteinogenic amino acids were synthesized under microwave irradiation using these reagents. Separation of diastereomeric pairs along with separation of a mixture of 30 diastereomers in a single chromatographic run was carried out on a reversed-phase C18 column. Mixtures of acetonitrile with aqueous trifluoroacetic acid were used as mobile phase. The detection was made at 230 nm using photo diode array detector. The separation behavior in terms of retention times and resolutions was compared on the basis of effect of chiral auxiliaries (i.e. amines) and achiral substituents (i.e. chlorine or butoxy group) in the chiral derivatizing reagents and the hydrophobic side chains of amino acids. The separation method was validated in terms of accuracy, precision, linearity, recovery, limit of detection and limit of quantitation. The method was successful for determination of d-amino acids in the absence of pure d-enantiomers and for separation of 19 diastereomers from a mixture of 30.



Similar content being viewed by others
References
Miyoshi Y, Koga R, Oyama T, Han H, Ueno K, Masuyama K, Itoh Y, Hamase K (2012) HPLC analysis of naturally occurring free d-amino acids in mammals. J Pharm Biomed Anal 69:42–49
Burton AS, Stern JC, Elsila JE, Glavin DP, Dworkin JP (2012) Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem Soc Rev 41:5459–5472
Bhushan R, Martens J (2010) Amino acids: chromatographic separation and enantioresolution. HNB Publishing, New York
Ilisz I, Aranyi A, Pataj Z, Péter A (2012) Recent advances in the direct and indirect liquid chromatographic enantioseparation of amino acids and related compounds: A review. J Pharm Biomed Anal 69:28–41
Marfey P (1984) Determination of d-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res Commun 49:591–596
Bhushan R, Kumar V (2008) Synthesis and application of new chiral variants of Marfey’s reagent for liquid chromatographic separation of the enantiomers of α-amino acids. Acta Chromatogr 20:329–347
Bhushan R, Kumar R (2009) Analysis of multicomponent mixture and simultaneous enantioresolution of proteinogenic and non-proteinogenic amino acids by reversed-phase high-performance liquid chromatography using chiral variants of Sanger’s reagent. Anal Bioanal Chem 394:1697–1705
Bhushan R, Brückner H (2004) Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247
Bhushan R, Brückner H (2011) Use of Marfey’s reagent and analogs for chiral amino acid analysis: assessment and applications to natural products and biological systems. J Chromatogr B 879:3148–3161
Bhushan R, Kumar V (2008) Indirect enantioseparation of α-amino acids by reversed-phase liquid chromatography using new chiral derivatizing reagents synthesized from s-triazine chloride. J Chromatogr A 1201:35–42
Bhushan R, Agarwal C (2011) Reversed-phase liquid chromatographic resolution of diastereomers of protein and non-protein amino acids prepared with newly synthesized chiral derivatizing reagents based on cyanuric chloride. Amino Acids 40:403–409
Bhushan R, Dixit S (2012) Application of cyanuric chloride-based six new chiral derivatizing reagents having amino acids and amino acid amides as chiral auxiliaries for enantioresolution of proteinogenic amino acids by reversed-phase high-performance liquid chromatography. Amino Acids 42:1371–1378
Bhushan R, Dixit S (2012) Amino acids as chiral selectors in enantioresolution by liquid chromatography. Biomed Chromatogr 26:962–971
Bhushan R, Dixit S (2012) HPLC enantioresolution of (R,S)-baclofen using three newly synthesized dichloro-s-triazine reagents having amines and five others having amino acids as chiral auxiliaries. Biomed Chromatogr 26:743–748
ICH-Topic Q2B (1996) Validation of analytical procedures. In: Proceedings of the international conference on harmonization of technical requirement for registration of pharmaceuticals for human use
Bhushan R (2011) Enantiomeric purity of chiral derivatizing reagents for enantioresolution. Bioanalysis 3:2057–2060
Bhushan R, Dixit S (2010) Reversed-phase high-performance liquid chromatographic separation of diastereomers of (R,S)-mexiletine prepared by microwave irradiation with four new chiral derivatizing reagents based on trichloro-s-triazine having amino acids as chiral auxiliaries and 10 others having amino acid amides. J Chromatogr A 1217:7669–7676
Bull HB, Breese K (1974) Surface tension of amino acid solutions. Hydrophobicity scale of the amino acid residues. Arch Biochem Biophys 161:665–670
Péter M, Fülöp F (2002) Comparison of isothiocyanate chiral derivatizing reagents for high-performance liquid chromatography. Chromatographia 56:631–636
Péter M, Péter A, Fülöp F (2000) Application of (1S, 2S)- and (1R, 2R)-1,3-diacetoxy-1-(4-nitrophenyl)-2-propylisothiocyanate to the indirect enantioseparation of racemic proteinogenic amino acids. J Chromatogr A 871:115–126
Péter A, Vekés E, Török G (2000) Application of (S)-N-(4-nitrophenoxycarbonyl) phenylalanine methoxyethyl ester as a new chiral derivatizing agent for proteinogenic amino acid analysis by high-performance liquid chromatography. Chromatographia 52:821–826
Suzuki T, Watanabe T, Toyo’oka T (1997) Discrimination of d/l-amino acid in peptide sequence based on fluorescent chiral derivatization by reversed-phase liquid chromatography. Anal Chim Acta 352:357–363
Kinoshita T, Kasahara Y, Nimura N (1981) Reversed-phase high performance liquid chromatographic resolution of nonesterified enantiomeric amino acids by derivatization with 2,3,4,6-tetra-Oacetyl-β-D-glucopyranosyl isothiocyanate and 2,3,4-tri-O-acetyl-β-D-glucopyranosyl isothiocyanate. J Chromatogr 210:77–81
Bhushan R, Kumar V, Tanwar S (2009) Chromatographic separation of enantiomers of non-protein α-amino acids after derivatization with Marfey’s reagent and its four variants. Amino Acids 36:571–579
Bank RA, Jansen EJ, Beekman B, te Koppele JM (1996) Amino acid analysis by reverse-phase high-performance liquid chromatography: improved derivatization and detection conditions with 9-fluorenylmethyl chloroformate. Anal Biochem 240:167–176
Einarsson S, Josefsson B (1987) Separation of amino acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl) ethyl chloroformate and reversed-phase liquid chromatography. Anal Chem 59:1191–1195
Acknowledgments
The authors are thankful to University Grants Commission (UGC) New Delhi, India, for awarding a senior research fellowship (SRF) (to M.L.). Thanks are due to AvH-Stiftung, Bonn, Germany for donating Knauer HPLC equipment (to R.B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhushan, R., Lal, M. LC Enantioseparation of 30-Component Diastereomeric Mixture of Amino Acids and Detection of d-Isomers Using New Reagents with Amines as Chiral Auxiliaries in Cyanuric Chloride. Chromatographia 76, 1087–1096 (2013). https://doi.org/10.1007/s10337-013-2515-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2515-6