Abstract
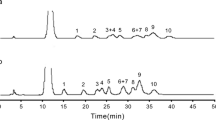
A reversed-phase high-performance liquid chromatographic method with fluorometric detection is proposed for the simultaneous determination of different classes of neutral sugars, such as hexoses (galactose, glucose and mannose), pentoses (arabinose and xylose), deoxy-hexoses (fucose and rhamnose), as well as acidic sugars (galacturonic and glucuronic acids). The separation is carried out on a hydrophilic end capped C18 column following a pre-column derivatization with p-aminobenzoic acid. The fluorometric detection of the derivatives has shown a strong dependency with the mobile phase pH. The performance of the proposed methodology was evaluated and the prerequisites of linearity (r-value > 0.999), precision (intra-day CV < 6 % and inter-day CV < 11 %) and recovery (between 77 ± 7 and 103 ± 3 %) were satisfied. To our knowledge, the obtained values of limit of detection for neutral sugars (within the range 6.1–28 μg L−1) are the lowest reported using this derivatizing agent. In order to better judge the methodology presented herein, neutral sugars of a pectin-rich orange extract were also analysed by the conventionally used GC-FID (gas-chromatography with flame ionization detector) method of alditol acetate derivatives. A statistical test (paired t test) has proved that no significant differences (α = 0.05) were observed between these two methods.




Similar content being viewed by others
References
Chassagne-Berces S, Poirier C, Devaux MF, Fonseca F, Lahaye M, Pigorini G, Girault C, Marin M, Guillon F (2009) Food Res Int 42:788–797. doi:10.1016/j.foodres.2009.03.001
Ralet MC, Lerouge P, Quemener B (2009) Carbohydr Res 344:1798–1807. doi:10.1016/j.carres.2008.08.036
Garna H, Mabon N, Wathelet B, Paquot M (2004) J Agr Food Chem 52:4652–4659. doi:10.1021/Jf049647j
Nunes C, Rato AE, Barros AS, Saraiva JA, Coimbra MA (2009) Food Chem 112:570–574. doi:10.1016/j.foodchem.2008.06.007
Nunes C, Santos C, Pinto G, Silva S, Lopes-Da-Silva JA, Saraiva JA, Coimbra MA (2009) Food Chem 115:1094–1101. doi:10.1016/j.foodchem.2008.12.089
Round AN, Rigby NM, MacDougall AJ, Morris VJ (2010) Carbohydr Res 345:487–497. doi:10.1016/j.carres.2009.12.019
Zhang Y, Huang LJ, Wang ZF (2007) Chinese J Chem 25:1522–1528. doi:10.1002/cjoc.200790280
Dai J, Wu Y, Chen SW, Zhu S, Yin HP, Wang M, Tang JA (2010) Carbohyd Polym 82:629–635. doi:10.1016/j.carbpol.2010.05.029
Lv Y, Yang XB, Zhao Y, Ruan Y, Yang Y, Wang ZZ (2009) Food Chem 112:742–746. doi:10.1016/j.foodchem.2008.06.042
Yang XB, Zhao Y, Wang QW, Wang HF, Mei QB (2005) Anal Sci 21:1177–1180. doi:10.2116/analsci.21.1177
Dahlman O, Jacobs A, Liljenberg A, Olsson AI (2000) J Chromatogr A 891:157–174. doi:10.1016/S0021-9673(00)00619-1
Arnous A, Meyer AS (2008) Food Bioprod Proc 86:79–86. doi:10.1016/j.fbp.2008.03.004
Ramirez SC, Carretero AS, Blanco CC, de Castro MHB, Gutierrez AF (2005) J Sci Food Agr 85:517–521. doi:10.1002/Jsfa.2010
Ducasse MA, Williams P, Meudec E, Cheynier V, Doco T (2010) Carbohyd Polym 79:747–754. doi:10.1016/j.carbpol.2009.10.001
Shen XD, Perreault H (1998) J Chromatogr A 811:47–59. doi:10.1016/S0021-9673(98)00238-6
Liu XJ, Ai N, Zhang HY, Lu MZ, Ji DX, Yu FW, Ji JB (2012) Carbohydr Res 353:111–114. doi:10.1016/j.carres.2012.03.029
Montero CM, Dodero MCR, Sanchez DAG, Barroso CG (2004) Chromatographia 59:15–30. doi:10.1365/s10337-003-0134-3
Burana-osot J, Soonthornchareonnon N, Chaidedgumjorn A, Hosoyama S, Toida T (2010) Carbohyd Polym 81:461–465. doi:10.1016/j.carbpol.2010.03.001
Anumula KR (2006) Anal Biochem 350:1–23. doi:10.1016/J.Ab.2005.09.037
Hase S (1996) J Chromatogr A 720:173–182. doi:10.1016/0021-9673(94)01166-4
Suzuki S, Fujimori T, Yodoshi M (2006) Anal Biochem 354:94–103. doi:10.1016/J.Ab.2006.04.013
Lamari FN, Kuhn R, Karamanos NK (2003) J Chromatogr B 793:15–36. doi:10.1016/S1570-0232(03)00362-3
Li JJ, Sun J, Wang ZF, Huang LJ (2010) Chromatographia 72:849–855. doi:10.1365/s10337-010-1755-y
Meyer A, Raba C, Fischer K (2001) Anal Chem 73:2377–2382. doi:10.1021/Ac001402s
Fischer K, Wacht M, Meyer A (2003) Acta Hydroch Hydrob 31:134–144. doi:10.1002/aheh.200300484
Gomis DB, Tamayo DM, Alonso JM (2001) Anal Chim Acta 436:173–180
Kurita O, Fujiwara T, Yamazaki E (2008) Carbohyd Polym 74:725–730. doi:10.1016/j.carbpol.2008.04.033
Westereng B, Michaelsen TE, Samuelsen AB, Knutsen SH (2008) Carbohyd Polym 72:32–42. doi:10.1016/j.carbpol.2007.07.017
Selvendran RR, March JF, Ring SG (1979) Anal Biochem 96:282–292. doi:10.1016/0003-2697(79)90583-9
Coimbra MA, Waldron KW, Delgadillo I, Selvendran RR (1996) J Agr Food Chem 44:2394–2401. doi:10.1021/Jf950637f
Moreira MM, Guido LF, Cruz JM, Barros AA (2010) Cent Eur J Chem 8:1236–1243. doi:10.2478/s11532-010-0101-4
Shuang SM, Yang Y, Pan JH (2002) Anal Chim Acta 458:305–310
Skoog DA, Holler FJ, Nieman TA (1998) Principles of instrumental analysis. Saunders College Publishing, USA
ICH QR (2005) Validation of Analytical Procedures: Text and Methodology
Miller JN, Miller JC (2000) Statistics and chemo metrics for analytical chemistry. Pearson Education Limited, Harlow
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, A.S., Valente, I.M., Nunes, C. et al. Determination of Aldoses, Deoxy-aldoses and Uronic Acids Content in a Pectin-Rich Extract by RP-HPLC-FLD after p-AMBA Derivatization. Chromatographia 76, 1117–1124 (2013). https://doi.org/10.1007/s10337-013-2510-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2510-y