Abstract
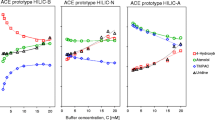
Computational models can be used to increase understanding of physical processes within chromatographic systems, leading to more efficient method development and optimisation strategies. In ion-exchange chromatography, various models have been derived to predict retention time; however, there remains a gap in understanding regarding the elucidation of fundamental processes contributing to retention. Here, artificial neural networks have been used to model retention of simple acidic analytes by strong anion-exchange HPLC in an attempt to understand what other factors aside from simple electrostatic interactions between ionised analyte, stationary phase and counter-ion contribute to the differential elution order of such compounds. The weights assigned by each neuron to the inputs in trained networks were used to infer the influence of a number of physicochemical analyte properties to retention under various conditions. These showed that several retention mechanisms were operating simultaneously, and that the contribution of each varied as eluent ionic strength and composition were altered at constant apparent pH. Analyte pKa had most influence on retention under most conditions, but analyte volume, LogP, and steric and electronic effects were also prominent, especially in eluents containing water.





Similar content being viewed by others
References
Buszewski B, Bocian S, Nowaczyk A (2010) J Sep Sci 33:2060–2068. doi:10.1002/jssc.201000101
Agatonovic-Kustrin S, Zecevic M, Zivanovic L (1999) J Pharm Biomed 21:95–103. doi:10.1016/S0731-7085(99)00133-8
Aschi M, D’Archivio AA, Maggi MA, Mazzeo P, Ruggieri F (2007) Anal Chim Acta 582:235–242. doi:10.1016/j.aca.2006.09.008
Kaliszan R, Baczek T, Bucinski A, Buszewski B, Sztupecka M (2003) J Sep Sci 26:271–282. doi:10.1002/jssc.200390033
Tham SY, Agatonovic-Kustrin S (2002) J Pharm Biomed 28:581–590. doi:10.1016/S0731-7085(01)00690-2
Wang Y, Zhang X, Yao X, Gao Y, Liu M, Hu Z, Fan B (2002) Anal Chim Acta 463:89–97. doi:10.1016/S0003-2670(02)00376-8
Zakaria P, Dicinoski G, Ng BK, Shellie RA, Hanna-Brown M, Haddad P (2009) J Chromatogr A 1216:6600–6610. doi:10.1016/j.chroma.2009.07.051
Zakaria P, Dicinoski G, Hanna-Brown M, Haddad PR (2010) J Chromatogr A 1217:6069–6076. doi:10.1016/j.chroma.2010.07.040
Agatonovic-Kustrin S, Zecevic M, Zivanovic L, Tucker IG (1998) J Pharm Biomed 17:69–76. doi:10.1016/S0731-7085(97)00170-2
Glass B, Agatonovic-Kustrin S, Chen Y, Wisch M (2007) J Chromatogr Sci 45:35–44
Havel J, Madden J, Haddad P (1999) Chromatographia 49:481–488
Marengo E, Gennaro MC, Angelino S (1998) J Chromatogr A 799:47–55. doi:10.1016/S0021-9673(97)01027-3
Sacchero G, Concetta Bruzzoniti M, Sarzanini C, Mentasti E, Metting HJ, Coenegracht PMJ (1998) J Chromatogr A 799:35–45. doi:10.1016/S0021-9673(97)01044-3
Novotná K, Havliš J, Havel J (2005) J Chromatogr A 1096:50–57. doi:10.1016/j.chroma.2005.06.048
Webb R, Doble P, Dawson M (2009) J Chromatogr B 877:615–620. doi:10.1016/j.jchromb.2009.01.012
Bolanca T, Cerjan Stefanovic S, Ukic S, Lusa M, Rogosic M (2009) Chromatographia 70:15–20. doi:10.1365/s10337-009-1126-8
Quiming N, Denola N, Saito Y, Jinno K (2007) Anal Bioanal Chem 388:1693–1706. doi:10.1007/s00216-007-1415-8
Quiming NS, Denola NL, Ueta I, Saito Y, Tatematsu S, Jinno K (2007) Anal Chim Acta 598:41–50. doi:10.1016/j.aca.2007.07.039
Al-Haj M, Kaliszan R, Buszewski B (2001) J Chromatogr Sci 39:29–38
Al-Haj MA, Kaliszan R, Nasal A (1999) Anal Chem 71:2976–2985. doi:10.1021/ac9901586
Kaliszan R, van Straten MA, Markuszewski M, Cramers CA, Claessens HA (1999) J Chromatogr A 855:455–486. doi:10.1016/S0021-9673(99)00742-6
Szepesy L (2002) J Chromatogr A 960:69–83. doi:10.1016/S0021-9673(02)00243-1
Gu RF, Jezorek JR (2001) J Chromatogr A 919:21–28. doi:10.1016/S0021-9673(01)00786-5
Morgan PE, Hanna-Brown M, Flanagan RJ (2006) Biomed Chromatogr 20:765–773. doi:10.1002/bmc.595
Nazir J, Barlow DJ, Lawrence MJ, Richardson CJ, Shrubb I (2002) Pharm Res 19:1130–1136. doi:10.1023/A:1019889907976
Richardson CJ, Barlow DJ (1996) J Pharm Pharmacol 48:581–591. doi:10.1111/j.2042-7158.1996.tb05978.x
Charton M (1981) In: Taft RW (ed) Progress in physical organic chemistry, vol 13. John Wiley & Sons, Inc., Hoboken
Charton M (1975) J Am Chem Soc 97:1552–1556. doi:10.1021/ja00839a047
Hansch C, Leo A, Taft RW (1991) Chem Rev 91:165–195. doi:10.1021/cr00002a004
Richardson CJ, Mbanefo A, Aboofazeli R, Lawrence MJ, Barlow DJ (1997) J Colloid Interface Sci 187:296–303. doi:10.1006/jcis.1996.4678
Law B, Hussain MA (2000) J Pharm Biomed 22:149–154. doi:10.1016/S0731-7085(99)00284-8
Reber Brown F, Draper WM (1989) J Chromatogr A 479:441–444. doi:10.1016/S0021-9673(01)83361-6
Flanagan RJ, Morgan PE, Spencer EP, Whelpton R (2006) Biomed Chromatogr 20:530–538. doi:10.1002/bmc.671
Couchman L, Morgan PE, Flanagan RJ (2011) Biomed Chromatogr 25:867–872. doi:10.1002/bmc.1530
Croes K, McCarthy PT, Flanagan RJ (1995) J Chromatogr A 693:289–306. doi:10.1016/0021-9673(94)01116-V
Morgan PE, Tapper J, Spencer EP (2003) J Chromatogr B 798:211–215. doi:10.1016/j.jchromb.2003.09.046
Carlucci G, D’Archivio AA, Maggi MA, Mazzeo P, Ruggieri F (2007) Anal Chim Acta 601:68–76. doi:10.1016/j.aca.2007.08.026
Jansen M, Kiwata J, Arceo J, Faull K, Hanrahan G, Porter E (2010) Anal Bioanal Chem 397:2367–2374. doi:10.1007/s00216-010-3778-5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgan, P.E., Barlow, D.J., Hanna-Brown, M. et al. Artificial Neural Network Modelling of the Retention of Acidic Analytes in Strong Anion-Exchange HPLC: Elucidation of Structure-Retention Relationships. Chromatographia 75, 693–700 (2012). https://doi.org/10.1007/s10337-012-2251-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-012-2251-3