Abstract
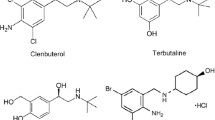
A clenbuterol (CLB) molecule-imprinted monolithic stationary phase (MIMSP) with specific recognition for CLB and some other β2-adrenergic receptor agonists was prepared by in situ polymerization technique utilizing methacrylic acid as a functional monomer, ethylene glycol dimethacrylate (EDMA) as a cross-linking reagent, and low polar solvents (toluene and dodecanol) as porogenic solvents. The optimal polymerization conditions were as follows: the molar ratio of template:monomer:initiator was 5:20:1, EDMA was 85% (v/v) in the total volume of monomer and EDMA, and toluene was 18% (v/v) in the total mixed porogen. The selectivity of the stationary phase for CLB and other β2-adrenergic receptor agonists was evaluated by high performance liquid chromatography. Scatchard analysis was employed to explore the recognition mechanism. Then the CLB-MIMSP was used as a solid phase extraction (SPE) stationary phase for concentration and purification of CLB from pig liver samples. The results showed that the obtained CLB-MIMSP possessed high selectivity towards CLB and moderate selectivity towards some other β2-adrenergic receptor agonists with characteristics of easy-made. The limit of detection was 10 ng g−1, and recoveries of CLB were 99.16–113.06% with RSD 4.55–11.81% for the spiked pig liver samples. The CLB-MIMSP could be a promising SPE absorbent in CLB biological sample pretreatment.








Similar content being viewed by others
References
Re G, Badino P, Novelli A, Girardi C (1997) Effects of clenbuterol as a repartitioning agent on [beta]-adrenoceptor concentrations in heart, bronchi and brain of veal calves. Vet J 153(1):63–70. doi:10.1016/s1090-0233(97)80009-3
Kearns CF, McKeever KH (2009) Clenbuterol and the horse revisited. Vet J 182(3):384–391. doi:10.1016/j.tvjl.2008.08.021
Waterfield CJ, Jairath M, Asker DS, Timbrell JA (1995) The biochemical effects of clenbuterol: with particular reference to taurine and muscle damage. Eur J Pharmacol Environ Toxicol Pharmacol Section 293(2):141–149. doi:10.1016/0926-6917(95)00010-0
Mazzanti G, Di Sotto A, Daniele C, Battinelli L, Brambilla G, Fiori M, Loizzo S, Loizzo A (2007) A pharmacodynamic study on clenbuterol-induced toxicity: [beta]1- and [beta]2-adrenoceptors involvement in guinea-pig tachycardia in an in vitro model. Food Chem Toxicol 45(9):1694–1699. doi:10.1016/j.fct.2007.03.002
Masci G, Casati G, Crescenzi V (2001) Synthesis and LC characterization of clenbuterol molecularly imprinted polymers. J Pharm Biomed Anal 25(2):211–217. doi:10.1016/s0731-7085(00)00477-5
Vlatakis G, Andersson LI, Muller R, Mosbach K (1993) Drug assay using antibody mimics made by molecular imprinting. Nature 361(6413):645–647
Haginaka J (2005) Selectivity of affinity media in solid-phase extraction of analytes. Trends Anal Chem 24(5):407–415
Sambe H, Hoshina K, Haginaka J (2007) Molecularly imprinted polymers for triazine herbicides prepared by multi-step swelling and polymerization method: their application to the determination of methylthiotriazine herbicides in river water. J Chromatogr A 1152(1–2):130–137. doi:10.1016/j.chroma.2006.09.003
Zhang QQ, Fu Q, Amut EJ, Fang Q, Zeng AG, Chang C (2009) Preparation and evaluation of propranolol-imprinted monolithic stationary phase by in situ technique and application in analysis of propranolol in biological samples. Anal Lett 42(3):536–554. doi:10.1080/00032710802677084
Brambilla G, Fiori M, Rizzo B, Crescenzi V, Masci G (2001) Use of molecularly imprinted polymers in the solid-phase extraction of clenbuterol from animal feeds and biological matrices. J Chromatogr B 759(1):27–32. doi:10.1016/s0378-4347(01)00199-2
Andrea P, Miroslav S, Silvia S, Stanislav M (2001) A solid binding matrix/molecularly imprinted polymer-based sensor system for the determination of clenbuterol in bovine liver using differential-pulse voltammetry. Sens Actuator B 76(1–3):286–294
Bruins CHP, Jeronimus-Stratingh CM, Ensing K, van Dongen WD, de Jong GJ (1999) On-line coupling of solid-phase extraction with mass spectrometry for the analysis of biological samples: I. determination of clenbuterol in urine. J Chromatogr A 863(1):115–122. doi:10.1016/s0021-9673(99)00959-0
Ou JJ, Kong L, Pan CS, Su XY, Lei XY, Zou HF (2006) Determination of dl-tetrahydropalmatine in Corydalis yanhusuo by l-tetrahydropalmatine imprinted monolithic column coupling with reversed-phase high performance liquid chromatography. J Chromatogr A 1117(2):163–169. doi:10.1016/j.chroma.2006.03.084
Yin J, Yang G, Chen Y (2005) Rapid and efficient chiral separation of nateglinide and its l-enantiomer on monolithic molecularly imprinted polymers. J Chromatogr A 1090(1–2):68–75. doi:10.1016/j.chroma.2005.06.078
Amut E, Fu Q, Fang Q, Liu R, Xiao AP, Zeng AG, Chang C (2010) In situ polymerization preparation of chiral molecular imprinting polymers monolithic column for amlodipine and its recognition properties study. J Polym Res 17(3):401–409. doi:10.1007/s10965-009-9326-3
Rao TP, Kala R, Daniel S (2006) Metal ion-imprinted polymers: novel materials for selective recognition of inorganics. Anal Chim Acta 578(2):105–116. doi:10.1016/j.aca.2006.06.065
Xu ZF, Kuang DZ, Feng YL, Zhang FX (2010) Combination of hydrophobic effect and electrostatic interaction in imprinting for achieving efficient recognition in aqueous media. Carbohydr Polym 79(3):642–647. doi:10.1016/j.carbpol.2009.09.010
Zhu Q-Z, Haupt K, Knopp D, Niessner R (2002) Molecularly imprinted polymer for metsulfuron-methyl and its binding characteristics for sulfonylurea herbicides. Anal Chim Acta 468(2):217–227. doi:10.1016/s0003-2670(01)01437-4
Haginaka J, Takehira H, Hosoya K, Tanaka N (1999) Uniform-sized molecularly imprinted polymer for (S)-naproxen selectively modified with hydrophilic external layer. J Chromatogr A 849(2):331–339. doi:10.1016/s0021-9673(99)00570-1
Xiang Y, Chen D (2007) Preparation of a novel pH-responsive silver nanoparticle/poly(HEMA-PEGMA-MAA) composite hydrogel. Eur Polym J 43(10):4178–4187. doi:10.1016/j.eurpolymj.2007.08.005
Verliefde ARD, Cornelissen ER, Heijman SGJ, Verberk J, Amy GL, Van der Bruggen B, van Dijk JC (2008) The role of electrostatic interactions on the rejection of organic solutes in aqueous solutions with nanofiltration. J Memb Sci 322(1):52–66. doi:10.1016/j.memsci.2008.05.022
Herraez-Hernandez R, Campins-Falco P (2000) Derivatization of ephedrine with o-phthaldialdehyde for liquid chromatography after treatment with sodium hypochlorite. J Chromatogr A 893(1):69–80
Vamvaca K, Jelesarov I, Hilvert D (2008) Kinetics and thermodynamics of ligand binding to a molten globular enzyme and its native counterpart. J Mol Biol 382(4):971–977. doi:10.1016/j.jmb.2008.07.049
van Zoelen EJJ, Kramer RH, van Moerkerk HTB, Veerkamp JH (1998) The use of nonhomologous scatchard analysis in the evaluation of ligand–protein interactions. Trends Pharmacol Sci 19(12):487–490
Acknowledgments
Financial support of this work by National Natural Science Foundations of China (No.30873193) to Qiang FU is gratefully acknowledged. The authors also thank professor Jun Haginaka from Mukogawa Women’s University, Japan, for his helpful discussion in molecularly imprinted polymers preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Y., Huang, P., Fu, Q. et al. Preparation of Monolithic Imprinted Stationary Phase for Clenbuterol by In Situ Polymerization and Application in Biological Samples Pretreatment. Chromatographia 74, 693–701 (2011). https://doi.org/10.1007/s10337-011-2129-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-011-2129-9